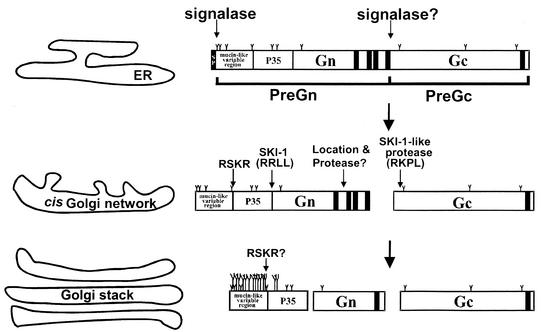
FIG. 7.
Proposed model of CCHF virus glycoprotein processing in mammalian cells. CCHF virus glycoprotein is likely synthesized as a precursor (its molecular size is not yet known). Upon translation, PreGn and PreGc are generated possibly after the hydrophobic domain and before RKPL, and this cleavage may be mediated by signal peptidase. From PreGc, mature Gc is processed following the RKPL (amino acid 1040) site, and this process is mediated by a protease other than SKI-1. This process is rapid as mature Gc can be visualized even during 20- or 30-min pulse periods. Mature Gc (but not PreGc) is recovered from the extracellular medium and becomes the structural element of the virus. Gn is generated from PreGn following the RRLL (amino acid 519) site, and this process is mediated by SKI-1. The processing of Gn appears to be a rather slow process, as Gn is visualized only in 2- to 3-h chase samples. Processing of Gn and Gc are not affected by BFA treatment, suggesting their occurrence in the ER-cis Golgi compartments. A 160-kDa protein (PreGn with terminal O-glycosidic modifications) and Gn are recovered from the extracellular media. CCHF virus contains predominantly mature forms of Gn and Gc as the glycoprotein elements. It remains to be seen if furin-mediated cleavage occurs at the RSKR (amino acid 247) site. If that happens, it will result in the separation of a mucin-like region and a protein with an expected molecular size of approximately 35 kDa (P35). The predicted O-glycosylation sites are indicated by long Y bars, and the predicted N-glycosylation sites are indicated by short Y bars.