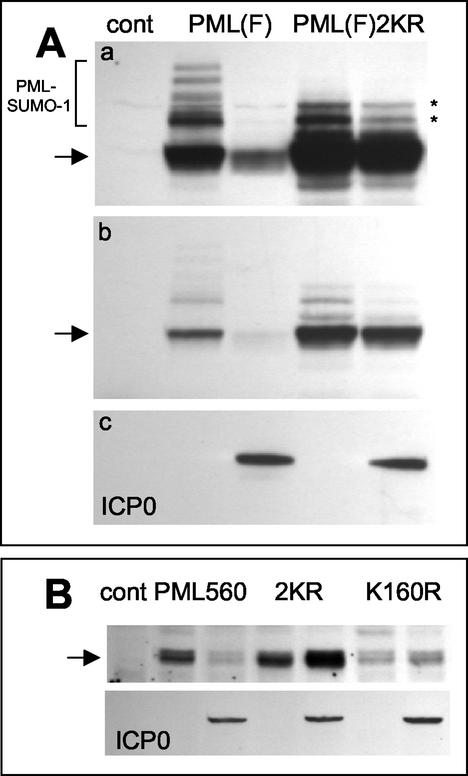
FIG. 8.
PML lysine residue 160, which is subject to conjugation to SUMO-1, is required for efficient ICP0-induced PML degradation. (A) Panel a, the left three lanes are equivalent to those in Fig. 7A, top panel, showing the modified (bracket) and unmodified (arrow) forms of full-length PML in the presence and absence of ICP0 as a control (cont). The right two lanes show the levels of expression of a PML derivative with lysine-to-arginine mutations at residues 160 and 490, the major characterized SUMO-1 modification sites of PML, in the presence and absence of coexpressed ICP0. The asterisks indicate uncharacterized modified forms of the mutant PML protein that migrate more rapidly than most of the SUMO-1-modified forms of the normal protein. Panel b, a shorter exposure of the same blot, showing the relative insensitivity of the double lysine mutant to ICP0 expression. Panel c, a reprobe of the same blot to detect ICP0 in the relevant lanes. (B) A similar experiment using the 560-residue PML isoform VI. The upper panel shows the control lane and then paired lanes of PML isoform VI (PML560), the double lysine mutant PML560SUMO- (2KR), and the single K160R mutant detected by Western blotting in extracts of cells cotransfected or not with an ICP0 expression plasmid (as detected in the lower panel by reprobing of the same filter). The amount of PML isoform VI was decreased by ICP0, whereas the amounts of the double and single lysine mutants were increased. The basal level of the single K160R mutant was lower than those of the other two proteins in this experiment.