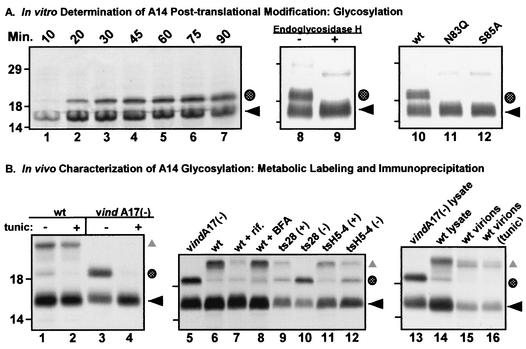
FIG. 3.
A highly conserved N-linked glycosylation motif found at the carboxy terminus of A14 is functional both in vitro and in vivo. (A) In vitro characterization of posttranslational modification of A14 by N-linked glycosylation. Lanes 1 to 7, IVTT time course of A14. A 50-μl IVTT reaction was programmed with pTM1-A14 and was performed in the presence of microsomal membranes according to the manufacturer's instructions. Five-microliter aliquots were removed at the times indicated (10 to 90 min) and were analyzed by SDS-PAGE (under reducing conditions) and autoradiography (lanes 1 to 7). The black arrow indicates the primary translation product; the shaded circle indicates a species of lower mobility that is predicted to represent a glycosylated form of A14. Lanes 8 and 9, removal of N-linked carbohydrate moiety from IVTT-synthesized A14 by EndoH. wt A14 synthesized in an IVTT reaction in the presence of microsomal membranes was subsequently incubated at 37°C for 24 h in the absence (lane 8) or presence (lane 9) of EndoH. EndoH treatment led to the disappearance of the slowly migrating form of A14 (shaded circle). Lanes 10 to 12, IVTT reactions programmed with alleles of A14 containing a disrupted N-linked glycosylation motif. Parallel IVTT reactions performed in the presence of microsomal membranes were programmed with plasmids encoding wt A14 (lane 10) or mutant alleles of A14 containing Asn83-to-Gln (lane 11, N83Q) or Ser85-to-Ala (lane 12, S85A) substitutions. Both of these amino acid substitutions prevent the appearance of the slowly migrating form of A14 (shaded circle). For all three panels, reactions were analyzed by SDS-PAGE and autoradiography; the positions of the 14,000-, 18,000-, and 29,000-Mr standards are shown at the left. (B) Determination of N-linked glycosylation status of A14 expressed in vivo. Lanes 1 to 4, low levels of glycosylated A14 are seen during wt virus infections. BSC40 monolayers were infected with either wt (lanes 1 and 2) or vindA17(−) virus (lanes 3 and 4) at an MOI of 10 in the presence (+) or absence (−) of tunicamycin (tunic). Cultures were metabolically labeled with [35S]methionine from 6 to 9 hpi; cell lysates were prepared and were subjected to immunoprecipitation with anti-A14 serum. Immunoprecipitates were resolved by SDS-PAGE and were visualized by autoradiography. ◂ indicates unmodified A14; the shaded circle indicates the putative glycosylated form of A14; ▴ indicates coprecipitated A17 protein. Lanes 5 to 12, early blocks to virion morphogenesis increase the levels of glycosylated A14. Cells were infected with either vindA17(−) (lane 5), wt virus (lane 6), wt virus plus rifampin (rif, lane 7), wt virus plus BFA (lane 8), ts28 at permissive (+) and nonpermissive (−) temperatures (lanes 9 and 10), or tsH5-4 at permissive (+) and nonpermissive (−) temperatures (lanes 11 and 12). Cells were metabolically labeled with [35S]methionine from 6 to 9 hpi; cell lysates were subjected to immunoprecipitation with anti-A14 serum. Immunoprecipitates were resolved by SDS-PAGE and were visualized by autoradiography. The symbols indicate the unmodified (◂) and glycosylated (shaded circle) forms of A14 as well as A14's binding partner, A17 (▴). Lanes 13 to 16, the glycosylated form of A14 is not present within virions. BSC40 cells were infected at an MOI of 2 with wt virus in the absence (lane 15) or presence (lane 16) of tunicamycin. Virions were harvested 48 hpi and were purified by ultracentrifugation through a 36% sucrose cushion followed by banding on a 25 to 40% sucrose gradient. Virions were disrupted and subjected to immunoprecipitation with anti-A14 serum. Immunoprecipitates were resolved by SDS-PAGE and were visualized by fluorography. Immunoprecipitates from vindA17(−)- and wt virus-infected cell lysates (lanes 13 and 14, respectively) served as controls for high and low levels of glycosylated A14 (shaded circle) as well as the coprecipitation of the A17 protein (▴). For all three panels, the positions of the 14,000-, 18,000-, and 29,000-Mr standards are shown at the left.