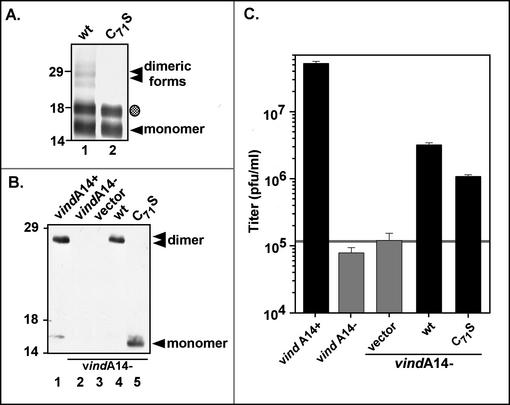
FIG. 6.
Cys71 is necessary and sufficient for intermolecular disulfide bond formation by A14, both in vitro and in vivo. (A) Synthesis of wt A14 (lane 1) and the C71S A14 mutant (lane 2) by using IVTT. [35S]methionine-labeled IVTT products were subjected to SDS-PAGE in the absence of reducing agents in order to assess the ability of the A14 proteins to undergo covalent dimerization; proteins were visualized by autoradiography. The unmodified (◂) and glycosylated (shaded circle) monomers of A14 were seen, as were several more slowly migrating forms that represent dimeric forms of wt A14 (stacked triangles). The positions of the 14,000-, 18,000-, and 29,000-Mr standards are shown at the left. (B) Immunoblot analysis of the wt and C71S A14 proteins expressed in vivo. Transient-complementation analysis was performed by using the infection-transfection protocol described above. Lysates were resolved by nonreducing SDS-PAGE; A14 species were visualized by immunoblot analysis. Infections with vindA14 were performed in the presence (lane 1) or absence (lanes 2 to 5) of TET. The latter were transfected with vector alone (lane 3) or plasmids encoding wt A14 (lane 4) or the C71S allele of A14 (lane 5). The A14 monomer ([◂]) and dimer (stacked triangles) are indicated; no dimer is seen in cells expressing the C71S allele of A14 (lane 5). The positions of the 14,000-, 18,000-, and 29,000-Mr standards are shown at the left. (C) Transient-complementation assay using A14 C71S. BSC40 monolayers were infected with vindA14 in the presence (+) or absence (−) of TET and were transfected with empty vector or plasmids expressing wt A14 or the C71S allele. Twenty-four-hour viral yield was determined by plaque assay; the horizontal grey line represents the titer obtained upon transfection of empty vector.