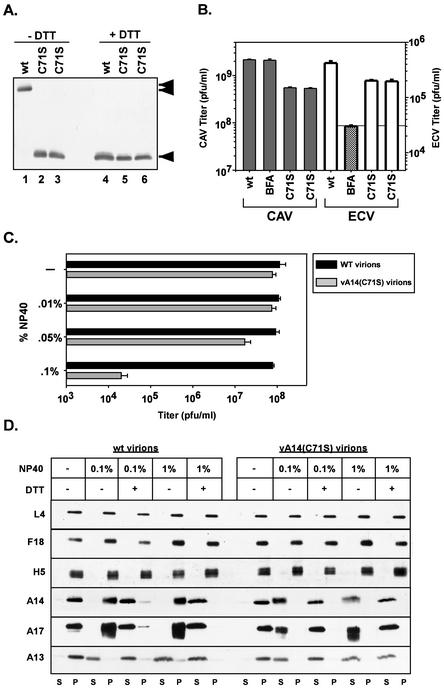
FIG. 7.
Characterization of vA14(C71S). (A) Immunoblot analysis of A14 encapsidated within purified wt or vA14(C71S) virions. One microgram of virions purified from cells infected with wt virus (lanes 1 and 4) or with two isolates of vA14(C71S) (lanes 2 and 5 and 3 and 6) was resolved by SDS-PAGE under nonreducing (lanes 1 to 3) or reducing (lanes 4 to 6) conditions and was subjected to immunoblot analysis with anti-A14 serum. The monomeric and dimeric forms of A14 are indicated (◂ and stacked triangles, respectively). (B) Determination of CAV and ECV yields. Cells were infected at an MOI of 2 with wt virus in the absence or presence of BFA or with vA14(C71S). Harvesting and titration of virus were performed as described for Fig. 4B and in Materials and Methods. Note the different scales used to plot the yields of CAV (left axis) and ECV (right axis). (C) Biological sensitivity of wt and vA14(C71S) virions to detergent treatment. A total of 2.0 × 108 wt or vA14(C71S) virions were incubated at 37°C for 30 min in various concentrations of NP-40 (0, 0.01, 0.05, or 0.1%) and were then titrated to determine the impact on viral titer (number of PFU/milliliter). (D) Permeabilization of wt and vA14(C71S) virions and fractionation of membrane and core components. wt and vA14(C71S) virions were subjected to detergent permeabilization and centrifugation to separate membrane and core components. Virions were incubated at 37°C for 30 min in 100 mM Tris (pH 9.0) containing NP-40 (0.1 or 1%) in the absence or presence of 50 mM DTT (− and +, respectively). After permeabilization, solubilized (S) and particulate (P) fractions were separated by sedimentation. Samples were resolved by SDS-PAGE, and the partitioning of various viral proteins was catalogued by immunoblot analysis by using sera directed against core (L4, F18, and H5) and membrane (A14, A17, and A13) proteins.