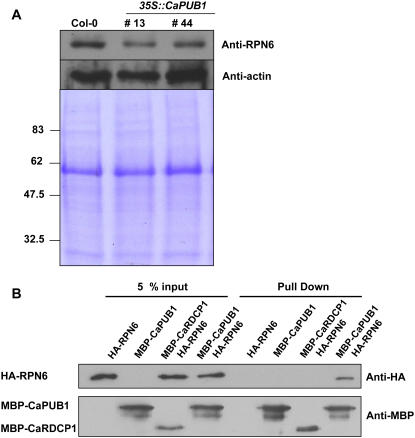
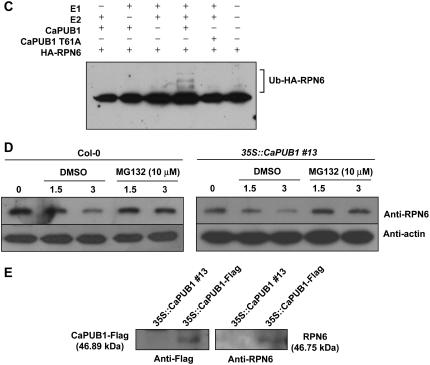
Figure 10.
CaPUB1 interacts with the RPN6 protein. A, Immunoblot analysis of RPN6 protein. Protein samples (20 μg of total proteins) were prepared from 7-d-old wild-type and two independent transgenic seedlings (lines 13 and 44), and subjected to immunoblot analysis using an anti-RPN6 antibody or anti-actin antibody as a negative control. The blot was visualized by the chemiluminescence detection method. The equivalence of protein loading among lanes of the SDS-PAGE gel was demonstrated by Coomassie Brilliant Blue R-250 staining of the proteins on the gel. B, In vitro pull-down assay. MBP-CaPUB1 and HA-RPN6 fusion proteins were coincubated with amylose affinity matrix. The bound protein was eluted from the amylose resin by 10 mm maltose, resolved by 12.5% SDS-PAGE, and transferred to a nitrocellulose membrane. The blot was probed with an anti-HA antibody or anti-MBP antibody. C, In vitro ubiquitination assay of RPN6 using CaPUB1 as the E3 Ub ligase. Recombinant HA-RPN6 protein was incubated in the presence or absence of Ub, ATP, E1, E2, and wild-type MBP-CaPUB1 or its mutant form (T51A), for the appropriate time periods, and subjected to immunoblotting using an anti-HA antibody. D, In vivo interaction of CaPUB1 and RPN6. The wild-type (left) and 35S∷CaPUB1 transgenic seedlings (right) were treated with 100 μm cycloheximide for different time periods (0–3 h) in the absence or presence of MG132, an inhibitor of the 26S proteasome. During incubation, changes in the level of RPN6 protein were monitored using an anti-RPN6 antibody. E, In vivo immunoprecipitation assay. Putative interacting proteins with CaPUB1 were immunoprecipitated from the 35S∷CaPUB1-Flag transgenic seedlings using an anti-Flag antibody. Immunoprecipitated proteins were subsequently analyzed by immunoblotting with an anti-RPN antibody. As a specificity control, 35S∷CaPUB1 seedlings were used in an identical experiment. [See online article for color version of this figure.]