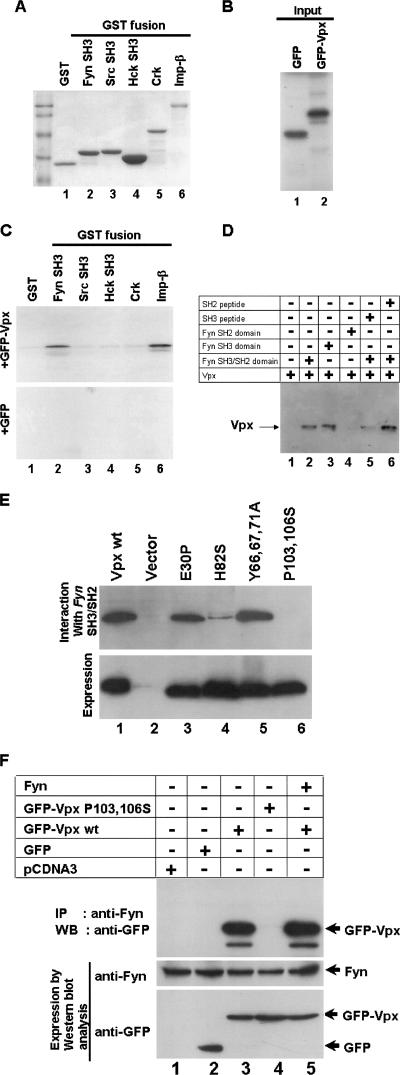
FIG. 3.
Evidence for Vpx interaction with the cellular Src-like tyrosine kinase Fyn. (A) SH3 domains of various kinases were expressed and purified as GST fusions. Glutathione-Sepharose beads containing equal amounts of GST and GST-SH3 fusion proteins as well as GST-importin-beta (GST-Imp-β) were used in the in vitro pulldown assays as indicated by the results of Coomassie blue staining. (B) [35S]methionine-labeled GFP and GFP-Vpx proteins (10%) were used in the GST pulldown assays. (C) Vpx interacts with Fyn SH3 domain. The results of GST pulldown assays using Fyn, Src, Hck, and full-length Crk as GST fusion proteins suggest that Vpx specifically interacts with the SH3 domain of Fyn. GST-importin-β was used as a positive control in this reaction. (D). Fyn peptides corresponding to the SH3 but not the SH2 domain block its interaction with Vpx. Cos-7 cell lysates containing Vpx were incubated with SH3 or SH2 domain peptides and mixed with glutathione-agarose beads containing Fyn SH3/SH2 domain. Bound proteins were resolved on SDS-15% PAGE followed by Western blot analysis using anti-Vpx monoclonal antibody. (E) Mutations within the COOH-terminus proline-rich motif abrogate Vpx interaction with the Fyn SH3 domain. Cos-7 cell lysates containing various mutant Vpx proteins were incubated with glutathione-agarose beads containing th e Fyn SH3/SH2 domain. Bound proteins were resolved on SDS-15% PAGE followed by Western blot analysis with anti-Vpx monoclonal antibody. (F) Vpx interaction with Fyn in vivo. GFP and GFP-Vpx (wild-type and P103 and 106S) expression plasmids were transfected in Cos-7 cells. After 16 h transfection, cell lysates were subjected to immunoprecipitation (IP) with anti-Fyn polyclonal antibody followed by Western blot analysis (WB) using anti-GFP monoclonal antibody. Expression of GFP-Vpx fusion proteins (5% input) and Fyn (10% input) was determined by Western blot analysis using anti-GFP monoclonal and anti-Fyn polyclonal antibodies, respectively.