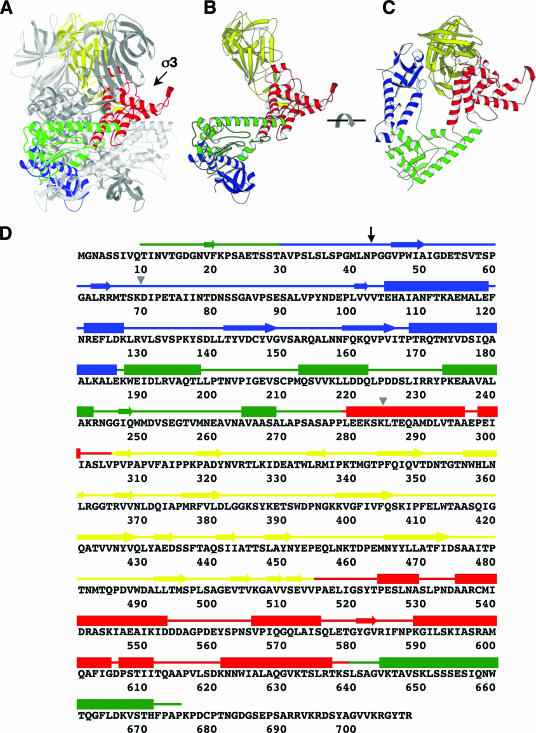
FIG. 1.
μ1 prior to structural rearrangement. (A) Side view of a μ1 trimer (as in the crystal structure of the μ13σ33 heterohexamer) without bound σ3, with one subunit highlighted (domain I, blue; domain II, green; domain III, red; domain IV, yellow). The other two μ1 subunits are in gray. One σ3 binding site is indicated by an arrow. (B) Side view of an isolated μ1 subunit, colored as described for panel A. (C) Top view of an isolated μ1 subunit, colored as described for panel A. (D) Amino acid sequence and secondary structure elements of reovirus μ1. The amino acid sequence of μ1 from reovirus serotype T1L is shown (5). Secondary structure elements, derived from the crystal structure of the μ13σ33 heterohexamer (15), are presented as tubes for α-helices and arrows for β-stands. The four domains of μ1 are colored as described above. The black arrow indicates the position of the autolytic cleavage, and the gray triangles indicate the starting and ending positions of the proteolysis-resistant fragment of μ1*.