Figure 8.
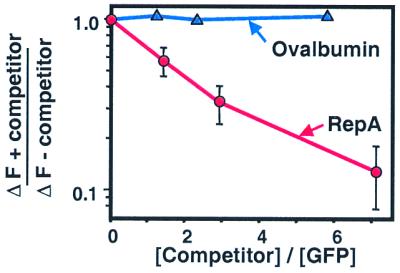
Competition between native and unfolded proteins for ClpA binding. ClpA (40 pmol) was incubated in 50-μl reaction mixtures containing buffer A with 2 mM ATP[γS] and 0, 60, 115, or 285 pmol of RepA (red circles) or 0, 50, 90, or 230 pmol of ovalbumin (blue triangles) for 2 min at 24°C. Then 40 pmol of acid-denatured GFP (in 50 μl) was added to each and fluorescence was measured after 10 min. Results are means (±SEM) of three independent experiments for RepA and a single representative experiment for ovalbumin.
