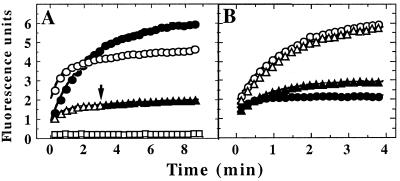
Figure 1.
Binding of specific unfolded proteins by ClpX. (A) GFP-SsrA was unfolded in acid and diluted to a final concentration of 0.5 μM in pH 7.5 buffer at 37°C with various additions; refolding was monitored by fluorescence expressed in arbitrary units. ●, No additions; ▵, 5 μM ClpX and 2 mM ATP[γS]; ○, 5 μM ClpX and 2 mM ATP[γS] with 8 μM λO added before GFP-SsrA; and □, 10 μM GroEL-trap. At the arrow, 8 μM λO was added to GFP-SsrA trapped with ATP[γS] and ClpX (▴. (B) GFP-UV (circles) or GFP-SsrA/DD (triangles) was denatured in acid and diluted into buffer with 2 mM ATP[γS] and either 5 μM ClpX (open symbols) or 5 μM ClpA (filled symbols). Both forms of GFP refolded with similar kinetics in the absence of ClpX and ClpA (data not shown).