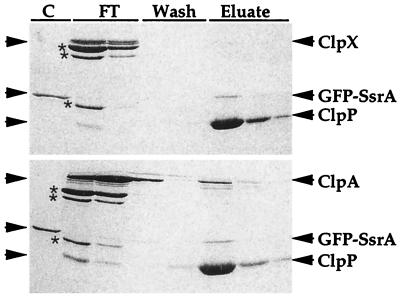
Figure 8.
Isolation of His6-ClpP-substrate complexes by metal-chelate chromatography. GFP-SsrA (1 μM) was incubated for 5 min at 37°C in 50 μl of buffer with 5 mM ATP and ClpXPin assembled with 1 μM ClpX and 1 μM His6-ClpP-CMK. The solution was diluted 20-fold in buffer without Mg and nucleotide and applied to a 200-μl bed of metal-chelate affinity gel. The column was washed once with 1 ml of buffer without Mg and nucleotide and twice with 1 ml each of the same buffer plus 10 mM imidazole. The bound protein was eluted with three washes with 200 μl of buffer with 200 mM imidazole. Proteins were precipitated with 10% trichloroacetic acid, prepared for SDS/PAGE, electrophoresed on a 10% polyacrylamide gel in SDS, and stained with Coomassie blue (Upper). The procedure was repeated but 1 μM ClpA was used instead of ClpX (Lower). Asterisks indicate proteins present in the creatine kinase added to reactions. Lane markings: C, GFP-SsrA; FT, flow-through of sample and buffer wash; Wash, 10 mM imidazole washes; Eluate, proteins eluted with 0.2 M imidazole.