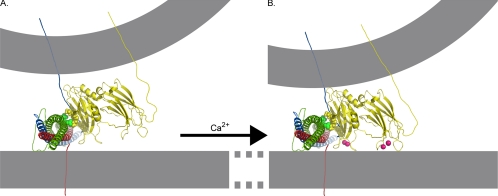
Figure 11.
Model of the role of the polylysine motif in Ca2+-independent synaptic vesicle docking/priming. Nuclear magnetic resonance structures of C2A (PDB file 1BYN) and C2B (PDB file 1K5W) domains (yellow) of synaptotagmin, the crystal structure of the core complex (PDB file 1SFC, containing VAMP [blue], SNAP-25 [green], and syntaxin [red]), and Ca2+ (pink) are shown to scale by using the PyMOL Molecular Graphics System (DeLano, 2002). The N termini of the SNARE proteins are darker and the C termini are paler to indicate depth. The membranes, the link between C2A and C2B domains of synaptotagmin, as well as transmembrane links for synaptotagmin, VAMP, syntaxin, and SNAP-25 were subsequently added. (A) Ca2+-independent interactions between the polylysine motif lysines in C2B (yellow, space-filled residues) with negatively charged residues of SNAP-25 (green, space-filled residues; Rickman et al., 2004, 2006) could hold the C2B Ca2+ binding site in the immediate vicinity of negatively charged phospholipids of the presynaptic membrane. (B) On Ca2+ influx, Ca2+-binding pockets of synaptotagmin insert into the plasma membrane (Bai et al., 2000, 2002, Wang et al., 2003; Rhee et al., 2005) allowing these negatively charged phospholipids to complete the coordination sphere for Ca2+ (Fernández-Chacón et al., 2002). The opposite end of the C2B domain interacts with the vesicle membrane (Araç et al., 2006) pulling the vesicle and plasma membranes closer together.