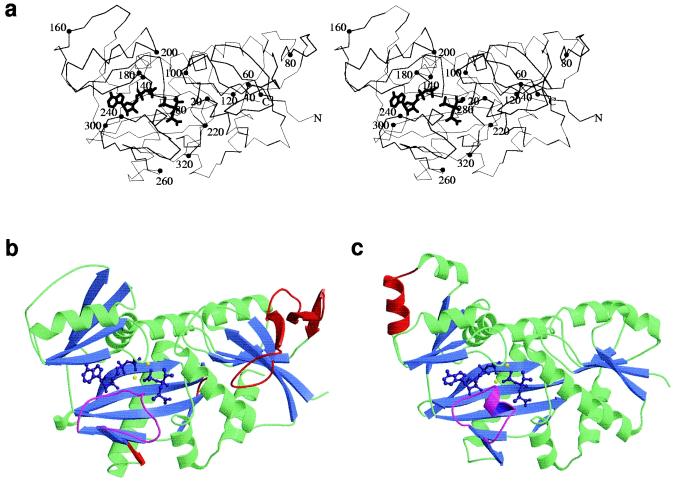
Figure 1.
Stereo C-α trace of VanA (a) with ADP and phosphophosphinate inhibitor showing overall structural features. Every twentieth residue is highlighted with a closed circle. N and C termini are marked N and C, respectively. In b and c, the structures of VanA and DdlB were overlaid in the program quanta and aligned by using close-residue optimization. Helices are displayed in green and β sheets in blue. ADP and phosphinophosphinate inhibitor are displayed in purple and magnesium ions in yellow. The ω-loops of both VanA and DdlB are displayed in magenta to highlight structural differences. Structural features corresponding to amino acid sequence found in one protein compared with the other are shown in red. In the structural alignment, a large additional loop occurs in VanA, between residues 44 and 90. The N terminus of each protein is shown on the right of each structure, immediately before the beginning of a β sheet found in both DdlB and VanA.