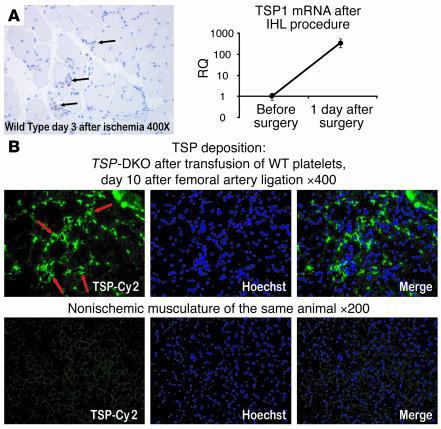
Figure 6. TSPs are deposited perivascularly in ischemic musculature.
(A) Left: WT mice underwent ligation of the left femoral artery. Shortly after the surgical procedure, ischemic tissue of the affected limb’s gastrocnemius muscle was harvested. Immunohistochemical analysis of the ischemic areas showed deposition of TSP in and around microvessels (black arrows). Note the perivascular location of freshly deposited TSPs along with inflammatory cells infiltrating the ischemic musculature. Importantly, there was no detectable staining on nonischemic musculature. Paraffin-embedded section from a representative field of WT gastrocnemius after femoral vessel ligation, stained with DAB and counterstained with hematoxylin. Scored at original magnification, ×400. IHL, ischemic hind limb. Right: RNA was extracted from ischemic gastrocnemius tissue, and TSP1 mRNA levels were compared with those at steady state. A strong increase in relative expression of TSP1 was found at the mRNA level (2–ΔCt × 10,000). This finding is in line with previous observations in skin healing models, where TSP1 mRNA is thought to be derived from invading hematopoietic cells. RQ, relative expression. (B) TSP-DKO mice 3 days after ischemic hind limb surgery received a single transfusion with 300 × 106 WT platelets. Seven days later, TSPs were deposited perivascularly only in ischemic musculature (red arrows). Frozen section, representative field of ischemic TSP-DKO gastrocnemius muscle, stained for TSPs with Cy2-conjugated secondary antibody and Hoechst 33342 nuclear stain. Scored at original magnification, ×400. As an internal control for autofluorescence and background staining, nonischemic musculature of the contralateral, nonischemic gastrocnemius muscle stained for TSP is shown. Original magnification, ×200.