Figure 4.
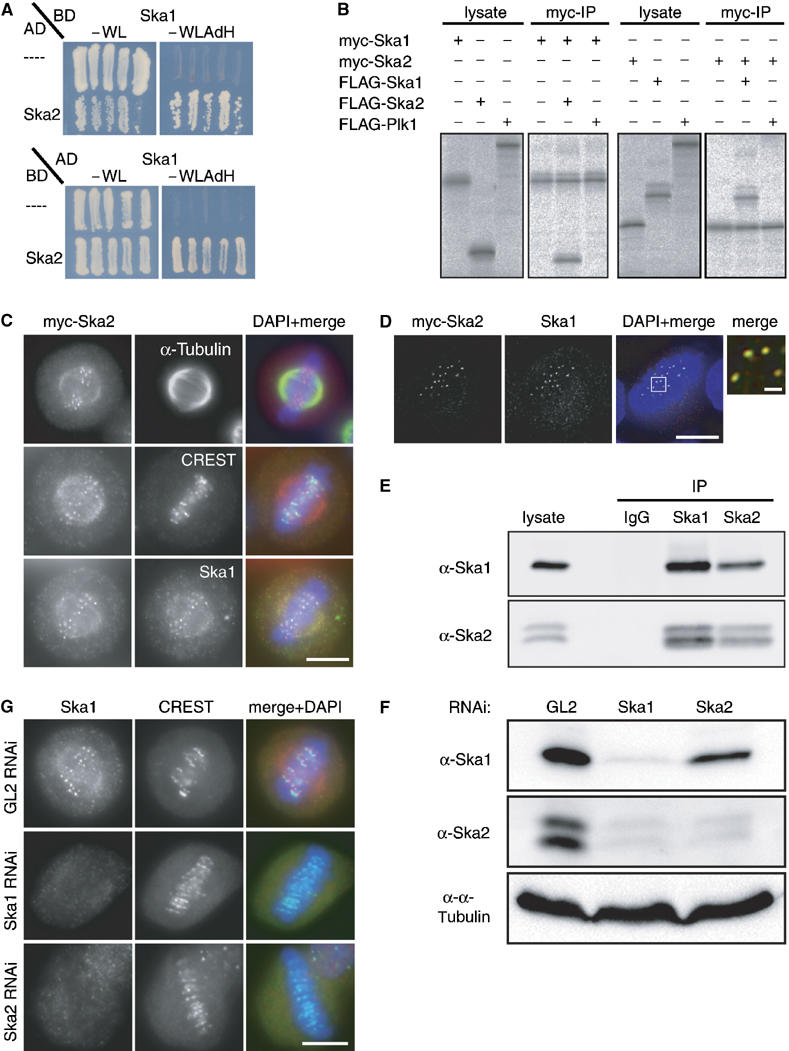
Ska1 interacts with another novel protein termed Ska2 (FAM33A). (A) Yeast two-hybrid interaction between Ska1 expressed as a binding domain (BD) fusion and Ska2 expressed as an activation domain (AD) fusion (top panel). In the bottom panel, Ska1 is expressed from the AD vector and Ska2 from the BD vector. As negative controls, the empty AD or BD (−) vectors were used. Interactions were reflected by growth on selective medium (−WLAdH, right panels). For control, growth on nonselective plates is also shown (−WL, left panels). (B) Myc- and FLAG-tagged versions of Ska1, Ska2 and Plk1 (negative control) were produced in different combinations by IVT in the presence of 35S-labelled methionine. Myc-tagged Ska1 (left panels) or Ska2 (right panels) were subsequently immunoprecipitated and IVT input and myc-immunoprecipitates were analysed by SDS–PAGE followed by autoradiography. (C) Myc-tagged Ska2 was transiently expressed in HeLa S3 cells for 48 h. After PTEMF fixation cells were stained with anti-myc 9E10 antibody (red), DAPI (DNA, blue) and with either anti α-tubulin antibody (upper panel), CREST serum (middle panel) or anti-Ska1 antibody (bottom panel) (all in green). (D) As in (C) after staining with anti-myc 9E10 antibody (red), anti-Ska1 antibody (green) and DAPI (DNA, blue), cells were imaged with a Deltavision microscope. Pictures are single, deconvolved focal planes. Right panel is a magnification of the marked area in the merged picture with scale bar indicating 1 μm. (E) Lysates were prepared from mitotic HeLa S3 cells. They were then used for immunoprecipitations with anti-Ska1 antibody, anti-Ska2 antibody and rabbit IgGs (negative control), respectively. Lysate and immune complexes were separated by SDS–PAGE and probed by Western blotting with anti-Ska1 and anti-Ska2 antibodies, as indicated. (F) HeLa S3 cells were treated for 48 h with control (GL2) and Ska1 and Ska2-specific siRNAs, respectively. Equal amounts of cell extracts were separated by SDS–PAGE and probed by Western blotting with anti-Ska1 and anti-Ska2 antibodies. Detection of α-tubulin was used as a loading control. (G) HeLa S3 cells were treated for 48 h with control (GL2), Ska1- and Ska2-specific siRNAs, respectively, then fixed with PTEMF and stained with anti-Ska1 antibody (red), CREST serum (green) and DAPI (DNA, blue). Scale bars=10 μm.
