Figure 2.
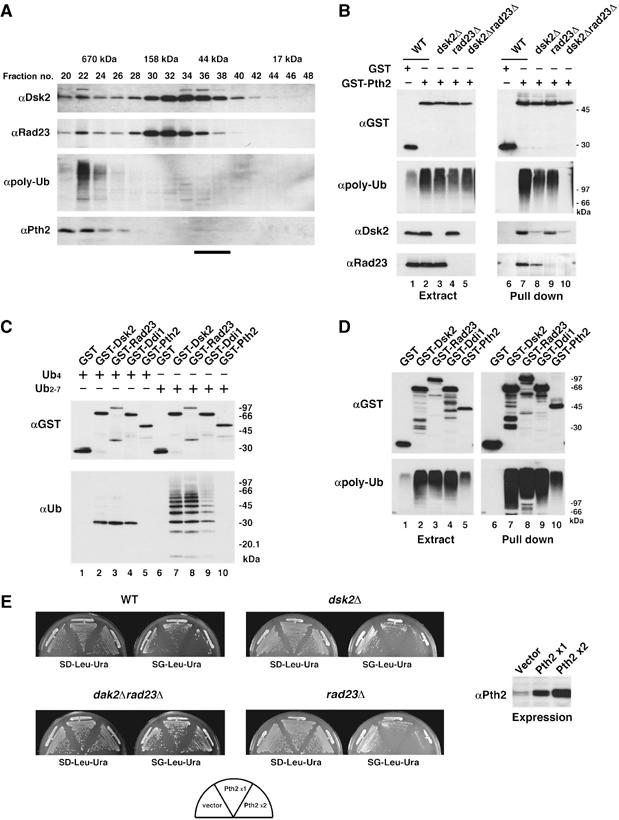
Pth2 associates with polyubiquitinated proteins via Dsk2 and Rad23. (A) Gel filtration of yeast extracts on a Superdex 200 column. Each fraction was immunoblotted with the indicated antibodies. The bar at the bottom indicates the position of monomeric Pth2 (∼25 kDa) (Rosas-Sandoval et al, 2002). (B) Binding of Pth2, Dsk2, Rad23 and polyubiquitin conjugates. GST-Pth2 was expressed in wild-type (YPH499), dsk2Δ, rad23Δ or dsk2Δrad23Δ strains. The GSH beads precipitates were immunoblotted as indicated. (C) In vitro GST pull-down assays with Pth2 and polyubiquitin. In total, 1 μg of tetraubiquitin (lanes 1–5) or polyubiquitin (lanes 6–10) was incubated with GST-Pth2, GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone (1 μg each). The samples were precipitated with GSH beads and immunoblotted as indicated. (D) In vivo GST pull-down assays with Pth2 and polyubiquitin. GST-Pth2, GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone was expressed in wild-type cells. Cell extracts were precipitated with GSH beads and immunoblotted as indicated. (E) Growth of yeast overexpressing Pth2. A single copy of PTH2 was expressed from a galactose-inducible pGAL1-YEplac181 ( × 1), or two copies of PTH2 were expressed from both pGAL1-YEplac181 and pGAL1-YEplac195 ( × 2) in wild-type, dsk2Δ, rad23Δ or dsk2Δrad23Δ strains. Cells were incubated for 2–5 days at 30°C, the permissive-temperature for growth of dsk2Δrad23Δ (Biggins et al, 1996). Expression levels were determined by Pth2 immunoblotting (on the right).
