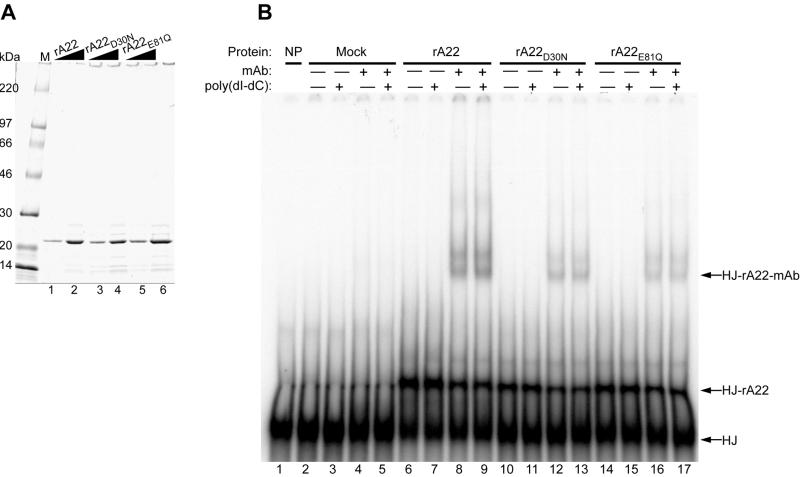
Figure 2.
Binding of affinity-purified rA22, rA22D30N, and rA22E81Q to a synthetic HJ. (A) Recombinant polyhistidine-tagged rA22, rA22D30N, and rA22E81Q were expressed in E. coli and purified by chromatography on a metal-affinity resin, analyzed on an SDS/4–20% polyacrylamide gel, and stained with Coomassie blue. Lanes: M, molecular mass markers; 1, 2.1 μg of rA22; 2, 4.2 μg of rA22; 3, 2.3 μg of rA22D30N; 4, 4.6 μg of rA22D30N; 5, 2.6 μg of rA22E81Q; and 6, 5.2 μg of rA22E81Q. Wedges indicate increasing amounts of protein. (B) Binding of rA22, rA22D30N, and rA22E81Q to the HJ. Affinity-purified recombinant proteins or mock-affinity-purified proteins from bacterial extracts were incubated with 0.1 pmol of HJ in the presence of EDTA, and with (+) or without (−) 75-fold excess poly(dI-dC)⋅poly(dI-dC) or anti-tetrahistidine monoclonal antibody (mAb). The native products were analyzed on a 4% polyacrylamide gel. Lanes: 1, no protein; 2–5, mock-affinity-purified proteins; 6–9, 0.42 μg of rA22; 10–13, 0.46 μg of rA22D30N; 14–17, 0.52 μg of rA22E81Q. Free HJ, HJ-rA22, and HJ-rA22-mAb complexes are indicated on the right side of the autoradiogram.