Abstract
Maternal hormones in vertebrate eggs can mediate important forms of maternal effects. However, the function of hormone transfer to the eggs is still debated, especially because long-term fitness consequences have been little studied. We investigated the effect of prenatal exposure to physiologically elevated yolk testosterone (T) levels on reproduction of female pheasants (Phasianus colchicus) in captivity. We found that females hatching from T-injected eggs (T-females) had a lower egg-laying rate than controls, and their eggs were more frequently infertile than those laid by control females. There were no effects of prenatal maternal treatment on egg size and yolk T concentration, but eggs carrying a female embryo laid by T-females had smaller yolks than eggs with a male embryo, while there was no sex difference in yolk size among the eggs laid by control females. Progeny sex ratio was unaffected by maternal treatment. These findings suggest that the transfer of high androgen levels to the eggs by the mother is constrained by complex trade-offs between direct effects on her daughters’ reproduction and by trans-generational differential consequences on male and female descendants.
Keywords: egg laying, fecundity, fertility, maternal effects, sex allocation, testosterone
1. Introduction
The finding that cleidoic eggs of vertebrates contain hormones of maternal origin that can affect growth and behaviour of the young (Schwabl 1993; Conley et al. 1997; Janzen et al. 1998; Lovern & Wade 2003; see reviews in Gil 2003; Groothuis et al. 2005a) has raised considerable interest among evolutionary biologists, owing to the possibility that these substances mediate important forms of maternal effects whereby mothers may adaptively adjust offspring phenotype to suit the prevailing environmental conditions (Mousseau & Fox 1998).
Hormones can have both activational and organizational effects on the physiology and development of vertebrates, and studies of birds have shown that deposition of maternal androgens in the eggs is affected by extrinsic factors, such as social environment, parasite infestation and mate attractiveness (e.g. Schwabl 1997; Gil et al. 1999; Tschirren et al. 2004), or intrinsic factors (e.g. female condition; Verboven et al. 2003; Gil et al. 2006). Several studies have investigated whether the experimental modification of egg androgen content—via the injection into the egg of physiological amounts of hormones—influences offspring phenotype, highlighting that the consequences for the progeny of raised androgen levels in the egg can vary according to the specific trait under scrutiny, as well as among species (e.g. Schwabl 1996; Lipar & Ketterson 2000; Sockman & Schwabl 2000; Eising et al. 2001; Groothuis et al. 2005b; Müller et al. 2005; Saino et al. 2006; von Engelhardt et al. 2006; see Groothuis et al. 2005a for a recent review). Most of these studies have dealt with the effects of egg hormones in the early life stages, whereas only few experiments have investigated the effects of altered exposure to androgens in adulthood or the carry-over effects on the following generation(s). Some of these studies have provided evidence that early exposure to high androgen levels enhances male ornaments, aggressive behaviour or success in competitive interactions (Strasser & Schwabl 2004; Eising et al. 2006), whereas others have shown negative effects on either primary or secondary sexual traits (Uller et al. 2005; Rubolini et al. 2006).
In this study, we address whether prenatal exposure to physiologically high yolk testosterone (T) levels has consequences for fecundity, egg size, yolk T content and egg fertility of female pheasants (Phasianus colchicus) during their first breeding season. The only previous study on this topic, on Chinese quails (Coturnix chinensis), reported that females experiencing high prenatal T levels laid smaller eggs (Uller et al. 2005), thus suggesting that prenatal T may have long-term, negative consequences on female fitness. In addition, we investigated whether exposure of mothers to high prenatal T levels affected the primary sex ratio of their progeny, and whether it differentially affected primary resource allocation and yolk T transfer to sons and daughters. Sex ratio of avian clutches has been observed to vary non-randomly according to both external and internal stimuli (reviewed in Pike & Petrie 2003), and recent studies suggest that hormones may mediate facultative sex ratio adjustment in bird species, high circulating T levels being associated with male-biased sex ratios (Veiga et al. 2004; Pike & Petrie 2006; Rutkowska & Cichon´ 2006).
2. Material and methods
(a) Model organism and general procedures
The ring-necked pheasant is a large (ca 1 kg), sexually dimorphic, polygynous galliform showing uniparental maternal care. Males form polygynous groups of up to 18 females, which lay 8–15 eggs per clutch and care for the highly precocial chicks (Cramp 1998) that reach sexual maturity at 1 year of age. Pheasants are commonly bred in captivity for hunting purposes, and the present experiment was carried out on a captive stock maintained at a game farm during 2004–2005. In spring 2004, we obtained a set of 800 unincubated eggs laid by 155 polygynous groups (ca seven females per group). These eggs were injected with 40 ng of T dissolved in 20 μl sterile sesame oil, corresponding to 1.67 s.d. of the mean total amount estimated for the yolk in a sample of 13 freshly laid reference eggs belonging to the same set of eggs that were injected (see electronic supplementary material). Control eggs were injected with 20 μl sterile sesame oil. We decided to manipulate yolk T because the biological role of egg androgens other than T is less clear (Groothuis et al. 2005a). A detailed description of the injection procedures, together with incubation, rearing and caging conditions, is reported in the electronic supplementary material (see also Romano et al. 2005; Rubolini et al. 2006). At seven months of age, 114 females (59 from T-injected eggs and 55 from control eggs) were randomly assigned to 15 indoor cages (with sloped floors and egg catchers) to establish polygynous groups, seven of which contained control females (‘control cages’ hereafter) and the remaining eight containing T-females (‘T cages’ hereafter). The number of females per cage never exceeded eight (range 6–8) and did not differ between treatments, either at the beginning or at the end of the experiment (Mann–Whitney U-test, p=0.23 and 0.63, respectively). Seven females of each treatment group died during the egg-laying period. Each cage contained one randomly selected male originating from the stock of control eggs.
In captivity, females lay eggs from March to the end of June. In this experiment, the first egg appeared on day 11 (March 1=day 1). The cages were checked for laid eggs at variable time-intervals (mean 1.5±0.1 s.e., range 1–4 days; monitoring was mostly every day or every second day, except at the beginning of the season when the egg-laying rate is low). We decided to stop monitoring egg laying on day 77, when females had laid on average their 24th egg (averaged across all cages), corresponding approximately to two natural-sized (i.e. one first and one replacement) average clutches per female (Cramp 1998). All the eggs (n=3707) were removed from egg catchers and weighed (to the nearest 0.01 g) with an electronic scale. From day 43 to 55, cages were checked daily. Starting on day 43, i.e. the day when females had laid on average their sixth egg (averaged across all cages), eggs laid every second day were collected, for a total of seven collection days (until day 55). The collected eggs were incubated for 5 days to allow embryo development (n=568 eggs), after which the embryo, if present, was removed and the yolk and the albumen fractions were separated and weighed (to the nearest 0.01 g). Embryos collected on days 49 and 53 (n=106) were sexed according to the protocols described by Griffiths et al. (1996), and weighed to the nearest 0.0001 g. Yolks were kept at −20°C for later analyses. All the yolks from fertile eggs collected on days 45, 49 and 53 were subjected to hormone assay (see §2b; n=162). In addition to the effect of treatment, we analysed the effect of incubation on yolk T levels (e.g. Elf & Fivizzani 2002) by comparing the T concentrations in 15 freshly laid eggs (one per cage, collected on day 50) and 30 embryonated eggs (two per cage, averaged for each cage for statistical analyses) collected the day before and the day after collecting the freshly laid eggs (days 49 and 51). A schematic diagram of the sampling scheme is given in the electronic supplementary material.
We also analysed whether yolk T levels differed between incubated eggs containing an embryo (n=28) and incubated eggs in a sample of eggs laid on the same day in a given cage that did not develop any embryo (n=25; 2–5 eggs per cage; since all the eggs from a given cage were laid on the same day and females do not lay more than one egg per day, they were treated as independent units in the analyses; see §3).
(b) Hormone assay
The baseline yolk T concentration of the injected eggs was estimated based on 13 freshly laid reference eggs (see §2a), according to the protocols described by Romano et al. (2005) and Rubolini et al. (2006). The hormone concentration of eggs laid by experimental females was assayed using a commercial radioimmunoassay kit from DSL Inc., Texas (DSL-4000; see electronic supplementary material for details and validation of the assay).
(c) Statistical analyses
Data were analysed using hierarchical linear models (Goldstein 1995) in the MLwiN v. 2.0.2 package. For all variables, two-level (binomial or normal) models were used, with cage as the highest-level unit (since eggs could not be assigned to individual females, females could not be used as independent units in the analyses) and the different days of egg collection (date) as replicate measurements, nested within cages. Cage was a random factor in all models, and, in addition, we included a random slope for date at the cage level when model fit was improved and the model converged. Fixed factors were treatment (0, control cages; 1, T cages), date, date2 (the latter except for egg composition, fertility and sex ratios, owing to the limited date interval and lack of model convergence in several cases) and the treatment×date interactions (when relevant). Additional details on model specifications are provided in §3 and the electronic supplementary material.
Since egg-laying activity was monitored at variable time-intervals (see §2a), we calculated an egg-laying index for each cage, expressed as: (number of eggs laid)/((number of females)×(number of days elapsed since last day of monitoring)). Data were pooled over periods of 3 or 4 days, which were regarded as cage replicates (see electronic supplementary material for details). Mean egg mass was calculated for each cage with the same time window as for the egg-laying index (3 or 4 days). Yolk and albumen masses were recorded for a subsample of eggs, during days 43–55 (see electronic supplementary material; figure 1). Data were included in the analyses as cage replicates. The proportion of fertile eggs was calculated only for days and cages where at least five eggs were available, in order to reduce the huge within-cage variability in the proportion of fertile eggs, which caused difficulties of model convergence for the random effects in explorative analyses. Sex ratio, expressed as the proportion of males, was calculated for each cage for the two collection days 49 and 53, which were considered as cage replicates.
Parameters with p>0.05 were removed successively from a full model (Crawley 1993), and all factors with p<0.05 were regarded as significant and retained in the final models. Means and parameter estimates are presented with their associated standard error.
3. Results
(a) Egg laying
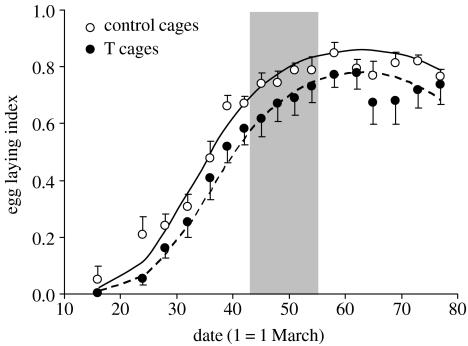
The mean egg-laying indeces were 0.62±0.02 (n=119 replicates) and 0.53±0.02 (n=136 replicates) for control and T cages, respectively, and showed a nonlinear increase during the sampling period (figure 1). After the removal of the non-significant treatment×date2 and treatment×date interactions (p=0.14 and 0.86, respectively), the hierarchical binomial model showed significant effects of both the linear and the quadratic terms of date (both p<0.001) and treatment (estimate=−0.54±0.24, χ2=5.11, p=0.024) on the egg-laying index. The lack of significant date by treatment interactions indicates that the seasonal increase of egg-laying activity did not differ between the two groups of cages (figure 1).
Figure 1.
Seasonal variation of the egg-laying index (see §2) among T and control cages. Circles represent the mean values for T and control cages and bars represent standard errors. Curves were obtained from a binomial hierarchical model (see §3). The shaded area (days 43–55) indicates the time window when eggs were collected for artificial incubation (see §2).
(b) Egg mass and composition
Mean egg mass varied according to a quadratic function of date (effect of date2: −0.0008±0.0001, χ2=28, p<0.001; effect of date: 0.082±0.017, χ2=22, p<0.001), with peak values in the middle of the sampling period, but this variation over time did not differ between treatments (treatment×date2, p=0.34; treatment×date, p=0.14), and egg masses of T (28.8±0.10 g, n=123 replicates) and control cages (28.6±0.10 g, n=113 replicates) were similar (p=0.80). There was no effect of maternal egg treatment on any of the measured egg composition variables (table 1; the treatment×date interaction was also never significant, all p>0.13).
Table 1.
Differences in egg composition between eggs laid by females from control eggs (control cages) and eggs laid by females from eggs injected with testosterone (T cages). (Sample size (n) refers to the number of cage replicates for each experimental group. χ2-values refer to δ deviance of hierarchical linear models. For further details see §2.)
| mean (s.e.; n) | ||||
|---|---|---|---|---|
| variable | control cages | T cages | χ2 | p |
| yolk mass (g) | 10.25 (0.08; 49) | 10.28 (0.11; 56) | 1.39 | 0.24 |
| albumen mass (g) | 10.50 (0.11; 49) | 10.51 (0.13; 56) | 0.11 | 0.74 |
| yolk T concentration (ng g−1) | 5.90 (0.18; 21) | 6.30 (0.18; 22) | 2.70 | 0.10 |
| total yolk T content (ng) | 59.30 (1.70; 21) | 61.19 (1.98; 22) | 0.78 | 0.38 |
Within each cage, yolk T concentration did not differ between the freshly laid and the embryonated eggs (5.66±0.34 versus 6.13±0.26 ng g−1, paired samples t-test, t14=−1.29, p=0.22), but incubated eggs that did not develop an embryo had higher T concentrations than embryonated ones, irrespective of maternal treatment (7.04±0.25 versus 6.24±0.23 ng g−1; two-level model of individual eggs (see §2) with cage as a level-2 variable, treatment, embryo presence and their interaction as predictors: effect of embryo presence, estimate=−0.79±0.32, χ2=5.68, p=0.017; effect of cage treatment, χ2=0.57, p=0.45; treatment×embryo presence, χ2=0.06, p=0.81). Qualitatively, the same effects were found for total yolk T content (details not shown).
(c) Egg fertility
The proportion of embryonated eggs was 65.6% (185/282) among T cages, whereas it was 75.9% (217/286) among control cages, a significant difference on a two-way contingency table (χ2=7.24, p=0.007). The difference was also significant in a two-level binomial model with the daily proportion of fertile eggs (calculated on a minimum of five eggs, see §2c) for each cage as the dependent variable (mean proportion, T cages=0.59±0.05, n=35 replicates; control cages=0.78±0.03, n=39 replicates; effect of treatment, estimate=−1.46±0.65, χ2=5.04, p=0.025). This model also showed that the proportion of fertile eggs increased with date (estimate=0.060±0.026, χ2=5.23, p=0.022) in a similar fashion among both cage groups (treatment×date, p=0.28).
(d) Sex ratio and sex differences in egg characteristics
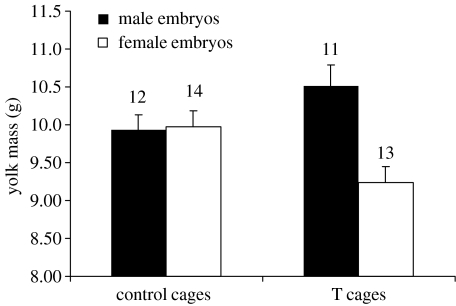
The sex ratio was male-biased in both treatment groups (T cages, 62.0% (31M : 19F); control cages, 67.9% (38M : 18F)). The sex ratio did not vary according to maternal egg treatment (estimate=−0.32±0.42, χ2=0.57, p=0.45). Sex differences in egg size, composition and embryo mass were analysed in two-level models where date was the level-1 and cage the level-2 variable, whereas cage treatment, embryo sex and their interaction were included as fixed predictors. Data for each sex category and collection day were averaged for each cage, in order to be included in the analyses as replicates (see §2c). There were no effects of cage treatment, embryo sex or their interaction on yolk T concentration or total yolk T content (all p>0.10, details not shown). However, T-females laid male eggs containing heavier yolks than female eggs, whereas yolk mass did not differ among male and female eggs laid by control females (figure 2; treatment×sex interaction: χ2=8.82, p=0.003). A similar trend was observed for egg mass (treatment×sex interaction: χ2=3.75, p=0.053), whereas there was no effect of cage treatment, sex or their interaction on albumen mass (all p>0.08). Finally, there were no effects of treatment, sex or their interaction on embryo mass (all p>0.39, details not shown).
Figure 2.
Yolk mass (mean+s.e.) of eggs containing male and female embryos in relation to maternal cage treatment. Numbers above bars represent sample size (number of replicates, see §2). The difference between male and female eggs among eggs laid by T-females was significant (two-level hierarchical linear model, χ2=11.9, p<0.001), as was the difference between female eggs laid by control and T-females (χ2=5.80, p=0.016). The difference between male eggs laid by control and T-females was marginally non-significant (χ2=2.74, p=0.098).
4. Discussion
Prenatal exposure of female pheasants to elevated yolk T levels negatively influenced egg production and reduced egg fertility during their first breeding season, whereas it did not affect either egg mass or composition, or the primary sex ratio. However, female eggs laid by T-females contained lighter yolks than male eggs. To our knowledge, this is the first study reporting that prenatal exposure to experimentally elevated androgen levels negatively affects fecundity and egg fertility in any bird species.
Interestingly, the negative organizational effects of prenatal exposure to elevated T levels on female fecundity are in line with previous studies showing negative activational effects of experimentally elevated circulating T levels in the adult bird on reproductive activities, such as lower egg-laying rates or failure to develop a brood patch (Clotfelter et al. 2004; Ketterson et al. 2005; Rutkowska et al. 2005). Our result may arise through an interference with factors regulating the ovulation–oviposition cycle. For example, females hatching from T-injected eggs may have increased endogenous T secretion, or have become more sensitive to the hormone. This may interfere with the hypothalamo–pituitary–gonadal axis, which controls the ovulation–oviposition cycle (Whittow 2000), and therefore reduce egg production. Alternatively, prenatal T may have altered ovarian development, with negative effects on later ovarian functionality and follicle maturation (e.g. Adkins-Regan et al. 1995).
The negative effects of prenatal androgen treatment on T-female fitness owing to reduced fecundity are exacerbated by lower egg fertility, i.e. a greater proportion of eggs that did not develop an embryo. It is unclear whether the negative effects of prenatal T exposure on egg fertility are due to a direct effect on the physiology of egg fertilization, or involves an indirect mechanism, such as a lower copulation frequency of males with T-females owing to a reduced sexual attractiveness of such females (see Ketterson et al. 2005). Intriguingly, infertile eggs had larger yolk T concentrations than eggs with developing embryos, irrespective of prenatal maternal treatment, confirming the same finding in zebra finches (Rutstein et al. 2005), but the lower fertility of eggs laid by T-females was not directly due to an increased yolk T concentration, as there was no difference in yolk T content according to maternal treatment of either embryonated or non-embryonated eggs. This result suggests that either an excess of T transfer during yolk deposition may prevent the subsequent egg fertilization and/or elevated T levels have toxic effects on the very early stages of embryonic development. This finding is unlikely to be due to the early androgen uptake by the embryo (Elf & Fivizzani 2002), as there was no difference in T concentration between the freshly laid and the embryonated eggs.
This study complements a previous one on the same species, where elevated yolk androgens were found to have no short-term effects on growth or immunity, but reduced the size of an important sexually selected trait of fully grown males, the length of tarsal spurs, and disrupted the natural covariation between the expression of sexual ornaments, therefore potentially altering the perception and assessment of multiple ornaments by females (Rubolini et al. 2006). Furthermore, female spurs were also shorter among T-females, although the function of female spurs is completely unknown (Rubolini et al. 2006). Therefore, these findings led us to hypothesize that transfer of maternal T to the eggs had negative effects on the phenotype of the male progeny and that directional selection towards lower yolk T transfer may occur in pheasants, and results of the present study, showing negative effects of prenatal T on female fitness, corroborate this idea.
A novel and important finding is that female eggs laid by T-females had smaller yolks than male eggs, while there was no sex difference in the yolk mass of eggs laid by control females. This was unlikely to be due to differential uptake of yolk material by embryos in relation to maternal egg treatment or sex, as embryo mass was unaffected by either treatment, sex or their interaction. Thus, prenatal exposure of mothers to T, besides reducing fecundity, may promote a differential allocation of resources to eggs in relation to the sex of the offspring, favouring sons over daughters by providing them with larger energy reserves than females. In fact, egg size, and yolk size in particular, is likely to represent an important determinant of early survival in the highly precocial galliform chicks, which mainly rely on yolk nutrients soon after hatching (Williams 1994; Finkler et al. 1998). Interestingly, there was a trend for antagonistic effects in relation to offspring sex, because daughters of T-females were provided with smaller yolks than daughters of control females, and sons of T-females tended to be provided with larger yolks than sons of control females (see legend to figure 2). This may indicate that females are faced with prenatal-T-mediated trade-offs on resource allocation to sons and daughters, suggesting that prenatal exposure of mothers to high T has trans-generational detrimental effects on the fitness of daughters, but positive effects on the fitness of sons. Indeed, a greater resource allocation to male compared with female offspring caused by prenatal T levels could be adaptive in the highly polygynous pheasant, and it could perhaps even offset the negative effects on mother's fitness owing to the lower egg-laying rate and egg fertility.
In conclusion, we have shown that prenatal exposure to high T levels negatively affected reproduction of female birds, by reducing egg-laying rates and egg fertility. This indicates that the transfer of androgens to the eggs by mothers involves long-term fitness costs, which apply to both males, through a reduced size of primary and secondary sexual characters (Uller et al. 2005; Rubolini et al. 2006), and females, via a negative effect on the reproductive performance. In addition, prenatal T may induce a sex-specific pattern of resource allocation to the eggs, whereby eggs containing daughters receive less resources than male eggs, thus raising the possibility that the deposition of high T levels in the eggs by mothers may mediate differential investment in male and female descendants by their daughters.
Acknowledgments
B. de Vries, M. Lasthuizen and C. Vermeulen kindly assisted with the hormone assay. We thank the many people and students who helped us with the data collection and laboratory analyses, and the referees for their constructive comments.
Supplementary Material
Detailed methodological explanation of egg injection procedures; detailed explanation of incubation procedures, animal housing and rearing; further detail on statistical methods; diagram that graphically shows the timing of egg sampling; detailed methodological explanation of yolk hormone assays; and references to articles cited within the ESM
References
- Adkins-Regan E, Ottinger M.A, Park J. Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. J. Exp. Zool. 1995;271:466–470. doi:10.1002/jez.1402710608 [Google Scholar]
- Clotfelter E.D, O'Neal D.M, Gaudioso J.M, Casto J.M, Parker-Renga I.M, Snajdr E.A, Duffy D.L, Nolan V, Jr, Ketterson E.D. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. doi:10.1016/j.yhbeh.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Conley A.J, Elf P, Corbin C.J, Dubowsky S, Fivizzani A, Lang J.W. Yolk steroids decline during sexual differentiation in the alligator. Gen. Comp. Endocrinol. 1997;107:191–200. doi: 10.1006/gcen.1997.6913. doi:10.1006/gcen.1997.6913 [DOI] [PubMed] [Google Scholar]
- Cramp S. Oxford University Press; Oxford, UK: 1998. The complete birds of the Western Palearctic on CD-ROM. [Google Scholar]
- Crawley M.J. Blackwell Science; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Eising C.M, Eikenaar C, Schwabl H, Groothuis T.G.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. doi:10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising C.M, Müller W, Groothuis T.G.G. Avian mothers create different phenotypes by hormone deposition in their eggs. Biol. Lett. 2006;2:20–22. doi: 10.1098/rsbl.2005.0391. doi:10.1098/rsbl.2005.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elf P.K, Fivizzani A.J. Changes in sex steroid levels in yolks of the Leghorn chicken, Gallus domesticus, during embryonic development. J. Exp. Zool. 2002;293:594–600. doi: 10.1002/jez.10169. doi:10.1002/jez.10169 [DOI] [PubMed] [Google Scholar]
- Finkler M.S, Van Orman J.B, Sotherland P.R. Experimental manipulation of egg quality in chickens: influence of albumen and yolk on the size and body composition of near-term embryos in a precocial bird. J. Comp. Physiol. B. 1998;168:17–24. doi: 10.1007/s003600050116. doi:10.1007/s003600050116 [DOI] [PubMed] [Google Scholar]
- Gil D. Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola. 2003;50:281–294. [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gil D, Marzal A, de Lope F, Puerta M, Møller A.P. Female house martins (Delichon urbica) reduce egg androgen deposition in response to a challenge of their immune system. Behav. Ecol. Sociobiol. 2006;60:96–100. doi:10.1007/s00265-005-0145-1 [Google Scholar]
- Goldstein H. Edward Arnold; London, UK: 1995. Multilevel statistical models. [Google Scholar]
- Griffiths R, Daan S, Dijkstra C. Sex identification in birds using two CHD genes. Proc. R. Soc. B. 1996;263:1251–1256. doi: 10.1098/rspb.1996.0184. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C.M. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005a;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Eising C.M, Dijkstra C, Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 2005b;1:78–81. doi: 10.1098/rsbl.2004.0233. doi:10.1098/rsbl.2004.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen F.J, Wilson M.E, Tucker J.K, Ford S.P. Endogenous yolk steroid hormones in turtles with different sex-determining mechanisms. Gen. Comp. Endocrinol. 1998;111:306–317. doi: 10.1006/gcen.1998.7115. doi:10.1006/gcen.1998.7115 [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166:S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. doi:10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovern M.B, Wade J. Yolk testosterone varies with sex in eggs of the lizard, Anolis carolinensis. J. Exp. Zool. 2003;295A:206–210. doi: 10.1002/jez.a.10225. doi:10.1002/jez.a.10225 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Müller W, Groothuis T.G.G, Kasprzik A, Dijkstra C, Alatalo R.V, Siitari H. Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc. R. Soc. B. 2005;272:1971–1977. doi: 10.1098/rspb.2005.3178. doi:10.1098/rspb.2005.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. doi:10.1017/S1464793103006146 [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc. R. Soc. B. 2006;273:1093–1098. doi: 10.1098/rspb.2005.3422. doi:10.1098/rspb.2005.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Rubolini D, Martinelli R, Bonisoli Alquati A, Saino N. Experimental manipulation of yolk testosterone affects digit length ratios in the ring-necked pheasant (Phasianus colchicus) Horm. Behav. 2005;48:342–346. doi: 10.1016/j.yhbeh.2005.03.007. doi:10.1016/j.yhbeh.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Rubolini D, Romano M, Martinelli R, Leoni B, Saino N. Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav. Ecol. Sociobiol. 2006;59:549–560. doi:10.1007/s00265-005-0080-1 [Google Scholar]
- Rutkowska J, Cichoń M. Maternal testosterone affects the primary sex ratio and offspring survival in zebra finches. Anim. Behav. 2006;71:1283–1288. doi:10.1016/j.anbehav.2005.07.025 [Google Scholar]
- Rutkowska J, Cichoń M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm. Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. doi:10.1016/j.yhbeh.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:62–69. doi:10.1093/beheco/arh123 [Google Scholar]
- Saino N, Ferrari R.P, Romano M, Martinelli R, Lacroix A, Gil D, Møller A.P. Maternal allocation of androgens and antagonistic effects of yolk androgens on sons and daughters. Behav. Ecol. 2006;17:172–181. doi:10.1093/beheco/arj023 [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. 1996;A114:271–276. doi: 10.1016/0300-9629(96)00009-6. doi:10.1016/0300-9629(96)00009-6 [DOI] [PubMed] [Google Scholar]
- Schwabl H. The contents of maternal testosterone in house sparrow Passer domesticus eggs vary with breeding conditions. Naturwissenschaften. 1997;84:406–408. doi: 10.1007/s001140050418. doi:10.1007/s001140050418 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. doi:10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 2004;56:491–497. doi:10.1007/s00265-004-0810-9 [Google Scholar]
- Tschirren B, Richner H, Schwabl H. Ectoparasite-modulated deposition of maternal androgens in great tit eggs. Proc. R. Soc. B. 2004;271:1371–1375. doi: 10.1098/rspb.2004.2730. doi:10.1098/rspb.2004.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller T, Eklöf J, Andersson S. Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav. Ecol. Sociobiol. 2005;57:584–590. doi:10.1007/s00265-004-0886-2 [Google Scholar]
- Veiga J.P, Viñuela J, Cordero P.J, Aparicio J.M, Polo V. Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm. Behav. 2004;46:47–53. doi: 10.1016/j.yhbeh.2004.01.007. doi:10.1016/j.yhbeh.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Verboven N, et al. Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus) Proc. R. Soc. B. 2003;270:2223–2232. doi: 10.1098/rspb.2003.2496. doi:10.1098/rspb.2003.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N, Carere C, Dijkstra C, Groothuis T.G.G. Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc. R. Soc. B. 2006;273:65–70. doi: 10.1098/rspb.2005.3274. doi:10.1098/rspb.2005.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittow G.C. Academic Press; London, UK: 2000. Sturkie's Avian physiology. [Google Scholar]
- Williams T.D. Intraspecific variation in egg size and egg composition: effects on offspring fitness. Biol. Rev. 1994;68:35–59. doi: 10.1111/j.1469-185x.1994.tb01485.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed methodological explanation of egg injection procedures; detailed explanation of incubation procedures, animal housing and rearing; further detail on statistical methods; diagram that graphically shows the timing of egg sampling; detailed methodological explanation of yolk hormone assays; and references to articles cited within the ESM