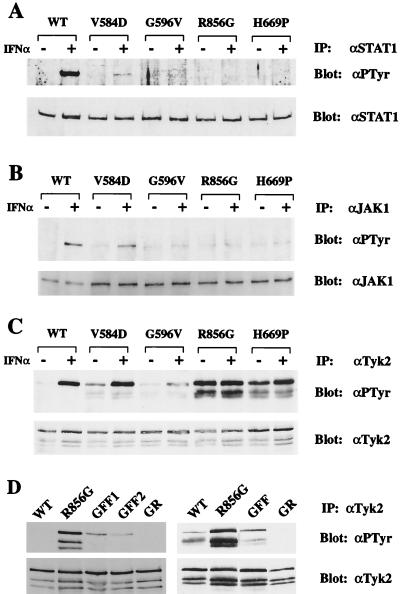
Figure 3.
In vivo phosphorylation levels of STAT1, JAK1, and Tyk2. (A) Cell lines expressing WT or mutant Tyk2 were treated with 103 IU/ml IFNα2 for 15 min. STAT1 was immunoprecipitated (IP) with an anti-STAT1 antibody (αSTAT1) and immunoblotted with an anti-phosphotyrosine antibody (αPTyr). The membrane was stripped and reprobed with an anti-STAT1 antibody to determine the level of immunoprecipitated STAT1. (B) JAK1 immunoprecipitates were immunoblotted with an anti-phosphotyrosine antibody. The membrane was stripped and reprobed with an anti-JAK1 antibody. In this experiment, more lysate was used from mutant-expressing cells than from WT cells to optimize the detection of phosphorylated JAK1. (C) Tyk2 immunoprecipitates were immunoblotted with an anti-phosphotyrosine antibody. The membrane was stripped and reprobed with an anti-Tyk2 antibody. The two lower bands, which have consistently been observed, correspond to Tyk2 degradation products. (D) Phosphorylation levels of the R856G/Y1054F/Y1055F and R856G/K930R mutants. (Left) Tyk2 was immunoprecipitated from stable clones expressing WT Tyk2, R856G, R856G/Y1054F/Y1055F (GFF, clones 1 and 2), or R856G/K930R (GR) proteins. Phosphorylation levels were detected by immunoblotting with an anti-phosphotyrosine antibody. The membrane was stripped and reblotted with an anti-Tyk2 antibody to determine protein levels. (Right) Immunoprecipitates of WT or mutant Tyk2 from transiently transfected 11,1 cells were analyzed as above.