Figure 5.
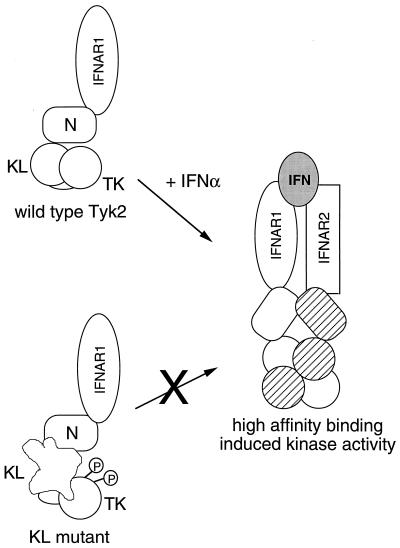
A model of Tyk2 regulation in the IFNα receptor-kinase complex. Tyk2 is associated with IFNAR1 through its N region. The KL domain maintains the TK domain in an unphosphorylated and resting conformation. Severe disruption of KL leads to hyperphosphorylation of the protein. Upon IFNα binding, KL-dependent conformational changes allow transition of the receptor-kinase complex to a high-affinity binding state where Tyk2 and JAK1 are stabilized in their activated conformations.