Abstract and Introduction
Abstract
Background
Early diagnosis of HIV infection provides the opportunity for treatment to prevent progression to AIDS and for intervention to prevent further transmission. The impact of routine screening of pregnant women and other factors on the stage of HIV disease at diagnosis were evaluated.
Methods
Data were collected in 1992–2002 from the medical records of persons presenting for HIV-related care at 2 major medical centers in Detroit, Michigan. Patients were included in the analysis if they had a CD4+ T-cell count recorded within 6 months of their first positive HIV test (N = 1858).
Results
Half of the patients (49%) had a first CD4+ T-cell count of < 200 cells/mm3 and 19% had an AIDS-defining illness at the time of HIV diagnosis. In the multivariate model, pregnant women were less likely than nonpregnant women to enter care with a CD4+ T-cell count of < 200 cells/mm3 (odds ratio, .24; 95% confidence interval, .14–.41). Even after adjusting for pregnancy, female sex was protective, as was age < 30 years. HIV-transmission risk factors, race, and time period of HIV diagnosis were not significantly associated with first CD4+ T-cell counts of < 200 cells/mm3.
Conclusion
Routinely offering HIV testing in prenatal care, as required by Michigan law, resulted in earlier diagnoses of HIV in pregnant women, as indicated by their higher CD4+ T-cell counts. Increasing routine HIV testing of all persons seeking medical care may increase the overall proportion of HIV diagnoses that are made early in the disease process.
Introduction
Increasing the proportions of HIV-positive persons who know their status and receive care is a strategic goal of the US Centers for Disease Control and Prevention (CDC).[1] As one of the tactics for achieving this goal, the CDC's recommendation on routinely offering HIV testing has recently been expanded to include all persons seeking healthcare in all clinical settings where the prevalence of HIV in the population is 1% or more.[2] Increasing the proportion of patients screened is expected to result in a larger proportion of HIV diagnoses being made at early stages of the disease. Early diagnosis of HIV benefits the patient because it provides the opportunity for treatment before the CD4+ T-cell counts drop below critical levels.[3–8] Early diagnosis of HIV also benefits the population because it provides the opportunity for intervention to decrease the risk of further transmission.[1,2]
Pregnant women in Michigan provided an opportunity to demonstrate the impact of routinely offering HIV testing. The Michigan law that requires healthcare providers to offer HIV testing to their pregnant patients at their first prenatal care visit, or at labor and delivery, became effective in 1989.[9,10] A high rate of compliance with this law was shown in 1998.[11] Approximately 80% of women who delivered live infants at a major medical center in Detroit reported being tested for HIV.[11] The data on pregnant women for the analysis presented here were collected at this same medical center, over a longer period, 1992–2002. The stage at which HIV infection was diagnosed in pregnant women was compared with other persons, for whom there was not a legal requirement for routinely offering HIV testing. This analysis also tested for associations of the year of first positive HIV test, sex, age, race, and HIV-transmission risk categories with the stage of HIV disease at diagnosis.
Methods
Population
Two observational databases were used for this analysis.
First, persons included in this analysis were selected from those enrolled in the Adult and Adolescent Spectrum of Disease (ASD) project. ASD is a national multisite, facility-based, longitudinal review of the medical records of HIV-infected persons, which was initiated in 1990.[12] Data for Michigan ASD were collected in Detroit at 2 large, urban medical centers, which provide primary and subspecialty care in outpatient clinics, emergency facilities, and hospitals, for a population of which 97% reside in Southeast Michigan. Prenatal care for 96% of the HIV-infected pregnant women in this analysis was provided at the same site used in the report on compliance with the Michigan statute on HIV testing of pregnant women.[11]
A stratified sample of all the persons who presented for HIV-related care at these sites were enrolled in ASD: all females age 13 and over, all males age 13–19 years, and a systematic random sample (40%) of new patients who were men aged >/= 20 years. The ASD database includes the date of the first positive HIV test recorded in the medical record, all CD4+ T-cell counts, and dates of pregnancy. Approval for ASD in Michigan was obtained from the institutional review boards of the Michigan Department of Community Health and the 2 medical centers where data were collected.
Second, data from the HIVAIDS Reporting System (HARS) were used to supplement the information in ASD, so that the earliest known positive HIV test date and the earliest known CD4+ T-cell count were used, even if these data were reported from a non-ASD site. HARS is the HIV/AIDS surveillance database in Michigan.[13] It includes the date of each person's first reported positive HIV test, the city and county of residence at the time of the first positive HIV test, and all reported CD4+ T-cell counts.
Inclusion Criteria
This analysis included the non-Hispanic black and white persons in ASD whose first reported positive HIV test occurred in 1992–2002, and whose first reported CD4+ T-cell count was within 6 months of their first reported positive HIV test. Persons in race categories other than black and white were excluded, because they constituted less than 5% of the ASD population and their numbers were too small to provide meaningful results.
Outcomes
First CD4+ T-cell count < 200 cells/mm, first CD4+ T-cell count < 50 cells/mm3, and definitive or presumptive diagnosis of any AIDS-defining illness[14] within a month before or after the first positive HIV test were used as indicators of advanced HIV disease. Any of these outcomes constitutes a diagnosis of AIDS, according to the CDC 1993 surveillance case definition.[14] In multivariate modeling, only the first CD4+ T-cell count of < 200 cells/mm3 was used as an outcome because of the comparatively small number of diagnoses of AIDS-defining illnesses.
Predictor Variables
Associations of stage of HIV disease with year of first positive HIV test, sex, race, age, pregnancy at the time of first CD4+ T-cell count, and mode of HIV transmission were studied. Age was divided into 2 groups, < 30 years and >/= 30 years, because in a preliminary analysis with 4 age categories, < 30, 30–39, 40–49, and >/= 50 years, the proportion of person with advanced HIV disease in the < 30 age group was significantly different from the others, whereas there were no significant differences among the 3 older age groups. The CDC-defined categories for mode of HIV transmission[15] were used with 1 modification. The heterosexual risk category included both the CDC-defined high-risk heterosexual category and the persons without an identified risk who had heterosexual contact.[16]
Data Analysis
Differences by year of first positive HIV test in the proportions of persons with CD4+ T-cell counts < 200 cells/mm3 within 6 months of their first positive HIV test, and in the proportions with diagnoses of any AIDS-defining illness[14] within 1 month of their first positive HIV test, were tested in logistic regressions without other covariates.
In univariate analyses, unadjusted odds ratios were calculated for first CD4+ T-cell counts < 200 cells/mm3 and for diagnoses of AIDS-defining illness, by sex, race, age group, pregnancy, and modes of HIV transmission.
Multivariate logistic regression models of first CD4+ T-cell counts < 200/mm3 over the predictor variables, sex, race, age group, pregnancy, HIV-transmission risk factors, and time period of first reported HIV test, were evaluated. The period of first reported HIV test was dichotomous, 1992–1995 vs 1996–2002, to compare outcomes before highly active combination antiretroviral therapy (HAART) was available with outcomes when HAART was available. Model fit was assessed with the −2 log likelihood method, and the most parsimonious model was selected. Interaction terms were not significant predictor variables and did not improve the fit of the model.
Software
Data analyses were performed with SAS/STAT Software (SAS Institute Inc., Cary, North Carolina) and Epi Info (CDC).
Results
The 2 medical centers where Michigan ASD data were collected provided HIV-related primary and subspecialty care for 69% of the 8307 residents of Southeast Michigan whose positive HIV status was reported in 1992–2002. Of the 2705 non-Hispanic persons with black or white race who were enrolled in ASD, 1858 met the additional inclusion criterion that their first CD4+ T-cell counts were within 6 months of their first positive HIV tests. The non-Hispanic persons with black or white race who did not meet this criterion were not significantly different from those included, with respect to sex, race, and age group (Table 1). However, injection drug users and persons with their first CD4+ T-cell counts >/= 200 cells/mm3 were more likely to be excluded than persons who did not have a history of injection drug use or whose first CD4+ T-cell counts were < 200 cells/mm3, respectively.
Table 1.
Demographic Characteristics and HIV-Transmission Risk Factors for Included vs Excluded Persons, Southeast Michigan, 1992–2002
| Included* (%) (n = 1858) | Excluded†† (%) (N = 847) | OR for Exclusion (95% CI)‡‡ | ||
|---|---|---|---|---|
| Sex | Man | 1111 (60) | 489 (58) | Referent |
| Woman | 747 (40) | 358 (42) | 1.09 (.92–1.28) | |
| Race | White | 348 (19) | 148 (17) | Referent |
| Black | 1510 (81) | 699 (83) | 1.09 (.88–1.34) | |
| Age | < 30 | 451 (24) | 183 (22) | Referent |
| >/= 30 | 1407 (76) | 664 (78) | 1.16 (.96–1.41) | |
| HIV-transmission risk factors§ | IDU | 558 (29) | 335 (40) | Referent |
| MSM | 701 (38) | 279 (33) | .65 (.54–.79) | |
| All heterosexual | 381 (21) | 149 (18) | .64 (.51–.81) | |
| Others | 228 (12) | 84 (10) | .60 (.45–.80) | |
| First CD4+ T-cell count | >/= 200 cells/mm3 | 945 (51) | 385 (56) | Referent |
| < 200 cells/mm3 | 913 (49) | 297 (44) | .80 (.67–.95) |
Included, first CD4+ T-cell count within 6 months of first reported positive HIV test
Excluded, first CD4+ T-cell count missing (165 persons) or more than 6 months after first reported positive HIV test (682 persons)
OR = odds ratio. The ratio of the odds of being excluded to the odds of being excluded in the referent group; 95% CI, 95% confidence interval. 95% CI's for sex, race, age, and first CD4+ T-cell count are from chi-square tests, and those for the transmission risk categories are from a logistic regression analysis of the transmission risk categories over the outcome. Results that are significant at the 95% level are in bold type.
IDU = injection drug users (including men who have sex with men as well as inject drugs); MSM = men who have sex with men; all heterosexual, both high-risk heterosexual (persons who have heterosexual contact with persons known to be HIV-positive or to have a risk factor for HIV infection) and presumed heterosexual (persons who have heterosexual contact and do not have any other known risk factors); others, persons with blood contact or no identified risk
Overall, 51% of the included persons had advanced HIV disease at the time of their first reported positive HIV tests, as indicated by one or more factors. Forty-nine percent had their first CD4+ T-cell counts < 200 cells/mm3; 27% had their first CD4+ T-cell counts < 50 cells/mm3; and 19% had diagnoses of an AIDS-defining condition within 1 month of the first reported positive HIV test.
These proportions did not change significantly over the period of 1992–2002. In any single year, 1993–2002, there were no significant differences compared with 1992 (for the various years, .05 < P < .69), and there were no significant linear trends over the time period (in chi-square tests for linear trend, .15 < P < .63).
A smaller proportion of women than men had advanced HIV disease at the time of diagnosis, and the proportion was even smaller among pregnant women than among their nonpregnant counterparts (Table 2). In the frequency distributions of the first CD4+ T-cell counts, it was evident that the counts for pregnant women were skewed toward higher values compared with nonpregnant women and men (Figure). For example, less than 3% of pregnant women had first CD4+ T-cell counts < 50 cells/mm3, in contrast to 25% of nonpregnant women and 31% of men (P < .0001). The mean first CD4+ T-cell count for pregnant women was the highest (475 ± 23.9 cells/mm3, mean ± standard error of the mean), followed by nonpregnant women (304 ± 11.9 cells/mm3), and then men (241 ± 7.5 cells/mm3).
Table 2.
Stage of HIV Disease at Time of First Positive HIV Test, Southeast Michigan, 1992–2002
| n | Proportion with CD4 < 200* | Unadjusted Odds Ratios for CD4 < 200 (95% CI)†† | Proportion with O. I.‡‡ | Unadjusted Odds Ratios for O. I. (95% CI) | ||
|---|---|---|---|---|---|---|
| Sex | Man (referent) | 1111 | 54% | .59 (.49–.71) | 23% | .52 (.40–.66) |
| Woman | 747 | 41% | 13% | |||
| Race | White (referent) | 348 | 49% | 1.03 (.81–1.30) | 19% | .98 (.73–1.32) |
| Black | 1510 | 49% | 19% | |||
| Age | < 30 (referent) | 451 | 35% | 2.09 (1.68–2.61) | 15% | 1.46 (1.09–1.95) |
| >/= 30 | 1407 | 54% | 20% | |||
| Pregnancy | Nonpregnant woman (referent) | 611 | 47% | .19 (.11–.33) | 16% | –§ |
| Pregnant woman | 120 | 14% | 0% | |||
| HIV-transmission risk factors| | IDU | 548 | 52% | Referent¶ | 18% | Referent¶ |
| MSM | 701 | 53% | 1.05 (.84–1.32) | 23% | 1.39 (1.05–1.85) | |
| All heterosexual | 381 | 39% | .60 (.46–.79) | 11% | .58 (.40–.86) | |
| Others | 228 | 47% | .84 (.61–1.14) | 24% | 1.50 (1.03–2.18) | |
| Overall | 1858 | 49% | 19% |
CD4 < 200, first CD4+ T-cell count < 200 cells/mm3
CI = confidence interval. 95% CI's are from chi-square tests; results significant at this level are in bold type.
O. I. = opportunistic illness, ie, diagnosis within a month of first positive HIV test of any AIDS-defining illness according to the 1993 US Centers for Disease Control and Prevention (CDC) surveillance case definition of AIDS.[13]
No pregnant women had a history of AIDS-defining illness.
IDU = injection drug users (including men who have sex with men as well as inject drugs); MSM = men who have sex with men; all heterosexual, both high-risk heterosexual (persons who have heterosexual contact with persons known to be HIV-positive or to have a risk factor for HIV infection) and presumed heterosexual (persons who have heterosexual contact and do not have any other known risk factors); others, persons with blood contact or no identified risk
Odds ratios and confidence intervals from the logistic regression comparing each transmission-risk category with the referent, IDU, without other covariates
Figure.
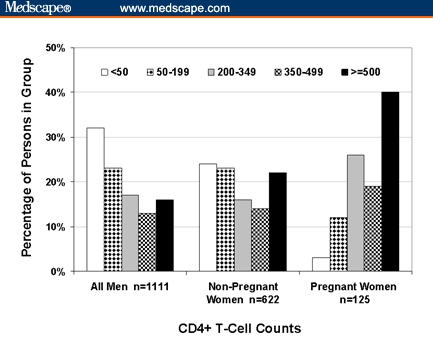
Frequency distributions of the first CD4+ T-cell counts of HIV-positive men, pregnant women, and nonpregnant women who had their first CD4+ T-cell counts within 6 months of their first reported positive HIV tests, Southeast Michigan, 1992–2002 (N = 1858). The CD4+ T-cell count categories shown in the legend are in cells/mm3.
Age < 30 years and heterosexual HIV-transmission risk were associated with earlier stages of HIV disease at diagnosis in the univariate analyses (Table 2). The unadjusted odds ratio for race was not significant at the 95% level.
Multivariate modeling of the outcome, CD4+ T-cell count < 200 cells/mm3, provided odds ratios for sex, race, age, and pregnancy that were adjusted simultaneously for all 4 of these predictor variables (Table 3). In the final model, female sex, age < 30 years, and pregnancy were associated with lower odds of having first CD4+ T-cell counts < 200 cells/mm3. Persons of white race had lower odds than persons of black race, although the odds ratios were not statistically significant at the 95% level. HIV-transmission risk factors and time period of first positive HIV test (1992–1995 vs 1996–2002) were not significantly associated with first CD4+ T-cell counts < 200 cells/mm3 in the multivariate model, and omitting them did not alter the fit of the model. Collinearity between pregnancy and age or sex did not affect the adjusted odds ratios or their confidence intervals, as shown in analyses of subgroups by sex and pregnancy (results not shown).
Table 3.
Multivariate Logistic Regression Model of First CD4+ T-Cell Count < 200 cells/mm3, Southeast Michigan, 1992–2002 (N = 1858)
| Predictor Variable | Value (Referent) | Adjusted Odds Ratio (95% CI)* |
|---|---|---|
| Sex | Woman (referent, Man) | .69 (.56–.85) |
| Race | Black (referent, White) | 1.25 (.98–1.60) |
| Age | >/= 30 (referent, < 30) | 1.81 (1.44–2.27) |
| Pregnancy | Pregnant (referent, not pregnant) | .24 (.14–.41) |
CI = confidence interval
95% C.I., 95% confidence interval. Results that are significant at the 95% level are in bold type.
Discussion
The utility of routine, voluntary screening in diagnosing HIV at an early stage was demonstrated in HIV-infected pregnant women presenting for testing and care. Their higher CD4+ T-cell counts compared with nonpregnant women and men were interpreted as indicating an earlier stage of HIV disease, not an effect of pregnancy on CD4+ T-cell counts, because pregnancy in HIV-infected women has not been shown to be associated with consistent changes in CD4+ T-cell counts compared with prepregnancy values.[17–24] The early diagnosis of HIV infection in pregnant women was consistent with previous reports that HIV-infected women with early, asymptomatic HIV disease were more likely to be pregnant than those who had advanced disease.[25–27] Because pregnant HIV-infected women were less likely to have advanced HIV disease, routine screening provided the opportunity to diagnose their HIV infections at an early stage.
The earlier diagnoses among pregnant women partially accounted for the earlier diagnoses among women compared with men, overall. Even after adjusting for pregnancy, however, the odds of having a first CD4+ T-cell count < 200 cells/mm3 were lower among women. This result was consistent with earlier reports from a variety of populations, times, and places, although these studies did not adjust for pregnancy.[28–35] One factor that contributed to this difference was that women in general have higher CD4+ T-cell counts than men. However, the 13% difference in mean CD4+ T-cell counts between HIV-negative women and men[36] did not entirely account for the 28% difference between the mean CD4+ T-cell counts of HIV-positive women and men in this analysis. Further study is needed to identify additional factors that may contribute to the higher CD4+ T-cell counts of HIV-infected women presenting for testing and care.
The results for age, race, and HIV-transmission risk factors were consistent with some previous reports but not others. The association of age >/= 30 years with higher odds of first CD4+ T-cell counts < 200 cells/mm3 has been reported,[28,32,33,37] although it has not been found in all studies.[38] The marginally significant association of black race with first CD4+ T-cell counts < 200 cells/mm3 in the multivariate model suggested that this population may be similar in this respect to the population studied at the University of Nebraska at Omaha, 1996–2001,[38] where a similarly marginal disparity was found in a multivariate analysis. In contrast, the unadjusted odds ratios did not show an association of race with later stages of HIV disease at diagnosis, in this analysis or in previous reports.[32,35] HIV-transmission risk factors not having statistically significant associations with CD4+ T-cell counts < 200 cells/mm3 in the multivariate model suggested that the significant unadjusted odds ratios were due to confounding, for example, by sex. Previous reports have varied in their conclusions about whether persons with different HIV-transmission risk factors presented for testing and care at different stages of disease.[32,33,37]
Limitations and Generalizability
ASD was representative of the HIV-infected population in Southeast Michigan (HARS) with respect to sex, race, age, and transmission risk factors (unpublished data). However, this analysis may have preferentially included persons who were at more advanced stages of HIV disease at the time of their first reported positive HIV tests than other HIV-infected persons in care in Southeast Michigan. One source of this bias was the preferential inclusion of persons with first CD4+ T-cell counts < 200 cells/mm3 that resulted from the requirement for a CD4+ T-cell count within 6 months of first reported positive HIV tests. This bias may have been compounded by preferential inclusion of persons with more advanced disease in the patient populations of the centers where ASD data were collected, because they provided specialized HIV care as well as primary care. In addition, a person's first positive HIV test might not have been reported, and they might have been inadvertently included in the analysis at a later stage of disease on the basis of their first positive HIV tests that were reported and followed by CD4+ T-cell counts within 6 months. However, the potential effects of these selection biases were limited because ASD represented such a large proportion of residents of Southeast Michigan whose positive HIV tests were reported to HARS.
The results of this analysis of Detroit data may not be generalizable to the rest of the state. Although compliance with the Michigan law requiring that HIV testing be routinely offered in prenatal care or in labor and delivery has been high in Detroit,[11] the same may not be true in other parts of the state.
Clinical and Public Health Significance
The incidence of AIDS and the numbers of HIV-related deaths have reached virtual plateaus since 1998, in contrast to the rapid declines that followed the introduction of HAART in 1996.[39,40] These plateaus can be partially accounted for by the lack of change over time in the proportion of HIV-infected persons who already had advanced disease when they were tested and entered care. This lack of change was found in previous reports from the United States, England, and Wales,[28,32] as well as in this analysis.
The substantial proportion of HIV-infected persons who presented for testing and care with CD4+ T-cell counts < 50 cells/mm3 and the lack of change in this proportion over time are of particular concern. Individuals with lower pretreatment CD4+ T-cell counts have been shown to have poorer responses to antiretroviral treatment.[4–8] These patients are less likely to attain a CD4+ T-cell counts above 200 cells/mm3 and, therefore, may be at prolonged risk of opportunistic diseases.
The proportion of HIV diagnoses made at an early stage of disease is expected to increase as the recent CDC recommendation to increase routine HIV screening[1,2] is implemented and the stigma attached to having an HIV test is decreased.[41] An increase in the proportion of persons tested when HIV testing was routinely offered has already been reported.[42,43] The additional persons tested, such as the pregnant women in this analysis, are expected to be at lower risk of having advanced HIV disease. Routine screening will be especially beneficial for persons over age 30, because they currently present for testing and care at later stages of HIV disease than younger persons.
The impact of earlier diagnosis on HIV-associated morbidity and mortality will depend on the rate at which the persons with newly diagnosed HIV infection enter care. The pregnant women in this analysis might have been highly motivated to enter care because of concern for their unborn children, and new approaches may be needed to increase the care-seeking motivation of other persons with newly diagnosed HIV infections.
Acknowledgments
The authors thank the Michigan Adult and Adolescent Spectrum of Disease (ASD) Data Abstraction Manager, Sharon Ritter; the ASD Data Abstractors, Karen Kaufmann, Wilma McGee, Meosia Lee-Turner, and Maureen Gargan, for collecting the data; and the Michigan ASD Data Assistant, Rosemary Parker Mackey, for entering the data into the ASD database. The authors also thank the staff of the HIV/AIDS Surveillance Section for the collection and entry of HIV case reports into HIVAIDS Reporting System (HARS).
Funding Information
The collection of ASD and HARS data and this analysis were supported by cooperative agreement number CCU 506 228 from the US Centers for Disease Control and Prevention (CDC), Atlanta, Georgia.
Contributor Information
Linda L. Wotring, Michigan Department of Community Health, Detroit, Michigan.
JoLynn P. Montgomery, Michigan Department of Community Health, Detroit, Michigan.
Eve D. Mokotoff, Michigan Department of Community Health, Detroit, Michigan.
Joseph N. Inungu, Michigan Department of Community Health, Detroit, Michigan.
Norman Markowitz, Henry Ford Health System, Detroit, Michigan.
Lawrence R. Crane, Adult HIV Program, Wayne State University School of Medicine, Detroit, Michigan.
References
- 1.US Centers for Disease Control and Prevention. HIV prevention strategic plan through 2005. 2001 Jan; Available at: http://www.cdc.gov/nchstp/od/news/prevention.pdf Accessed February 10, 2005.
- 2.US Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic – United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–332. [PubMed] [Google Scholar]
- 3.Yeni PG, Hammer SM, Carpenter CJ, et al. Antiretroviral treatment for adult HIV infection in 2002. JAMA. 2002;288:222–235. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann GR, Bloch M, Finlayson R, et al. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 2002;16:359–367. doi: 10.1097/00002030-200202150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Floridia M, Fragola V, Galluzzo CM, et al. HIV-related morbidity and mortality in patients starting protease inhibitors in very advanced HIV disease (CD4 count of < 50 cells/microL): an analysis of 338 clinical events from a randomized clinical trial. HIV Med. 2002;3:75–84. doi: 10.1046/j.1468-1293.2002.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Mokotoff ED, Malamud BH, Kent JB, et al. Progress toward elimination of perinatal HIV infections – Michigan, 1993–2000. MMWR Morb Mortal Wkly Rep. 2002;51:93–97. [PubMed] [Google Scholar]
- 10. Michigan Compiled Laws, Sec. 333.5123(1)
- 11.Schuman P, Jones TB, Ohmit S, et al. Voluntary HIV counseling and testing of pregnant women – an assessment of compliance with Michigan public health statutes. Medscape General Medicine. 2004;6(2) Available at: http://www.medscape.com/viewarticle/475007 Accessed February 3, 2005. [PMC free article] [PubMed] [Google Scholar]
- 12.Farizo KM, Buehler HW, Chamberland ME, et al. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992;267:1798–1805. [PubMed] [Google Scholar]
- 13.US Centers for Disease Control and Prevention. Atlanta, Ga: US Centers for Disease Control and Prevention; 1995. HIV/AIDS Reporting System (HARS) User Manual: Version 2.0. [Google Scholar]
- 14.US Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:RR–17. [PubMed] [Google Scholar]
- 15.US Centers for Disease Control and Prevention. Estimated number of adult/adolescent AIDS cases, by sex, exposure category and year of diagnosis, United States. HIV/AIDS Surveillance Report. 2000;12:1–44. Available at: http://www.cdc.gov/hiv/stats/hasr1202.pdf Accessed February 10, 2005. [Google Scholar]
- 16.Schmidt MA, Mokotoff ED. HIV/AIDS surveillance and prevention: improving the characterization of HIV transmission. Public Health Rep. 2003;118:197–204. doi: 10.1093/phr/118.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomala RE, Kalish LA, Zorilla C, et al. Women and Infants Transmission Study. Changes in total, CD4+, and CD8+ lymphocytes during pregnancy and 1 year postpartum in human immunodeficiency virus-infected women. Obstet Gynecol. 1997;89:967–974. doi: 10.1016/s0029-7844(97)00129-4. [DOI] [PubMed] [Google Scholar]
- 18.Vimercati A, Greco P, Lopalco PL, et al. Immunological markers in HIV-infected pregnant and non-pregnant women. Eur J Obstet Gynecol Reprod Biol. 2000;90:37–41. doi: 10.1016/s0301-2115(99)00228-6. [DOI] [PubMed] [Google Scholar]
- 19.Weisser M, Rudin C, Battegay D, et al. Swiss HIV Cohort Study and the Swiss Collaborative HIV and Pregnancy Study. Does pregnancy influence the course of HIV infection? J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:404–410. doi: 10.1097/00042560-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Alliegro M, Dorrucci M, Phillips AN, et al. Italian Seroconversion Study Group. Incidence and consequences of pregnancy in women with known duration of HIV infection. Arch Intern Med. 1997;157:2585–2590. [PubMed] [Google Scholar]
- 21.Buskin SE, Diamond C, Hopkins SG. HIV-infected pregnant women and progression of HIV disease. Arch Intern Med. 1998;158:1277–1278. doi: 10.1001/archinte.158.11.1277. [DOI] [PubMed] [Google Scholar]
- 22.Saada M, Le Chenadec J, Berrebi A, et al. SEROGEST and SEROCO Study Groups. Pregnancy and progression to AIDS: results of the French prospective cohorts. AIDS. 2000;14:2355–2360. doi: 10.1097/00002030-200010200-00017. [DOI] [PubMed] [Google Scholar]
- 23.Newell M-L, Rudin C, Cunn D, Peckham C. Immunological markers in HIV-infected pregnant women. The European Collaborative Study and the Swiss HIV Pregnancy Cohort. AIDS. 1997;11:1859–1865. [PubMed] [Google Scholar]
- 24.vanBenthem BHB, Vernazza P, Coutinho RA, et al. European Study on the Natural History of HIV Infection in Women and the Swiss HIV Cohort Study. The impact of pregnancy and menopause on CD4 lymphocyte counts in HIV-infected women. AIDS. 2002;16:919–924. doi: 10.1097/00002030-200204120-00012. [DOI] [PubMed] [Google Scholar]
- 25.Gray RH, Wawer MJ, Serwadda D, et al. Population-based study of fertility in women with HIV-infections in Uganda. Lancet. 1998;351:98–103. doi: 10.1016/S0140-6736(97)09381-1. [DOI] [PubMed] [Google Scholar]
- 26.Blair JM, Hanson DL, Jones JL, et al. Trends in pregnancy rates among women with human immunodeficiency virus. Obstet Gynecol. 2004;103:663–668. doi: 10.1097/01.AOG.0000117083.33239.b5. [DOI] [PubMed] [Google Scholar]
- 27.Ross A, Van der Paal L, Lubega R, et al. HIV-1 disease progression and fertility: the incidence of recognized pregnancy and pregnancy outcome in Uganda. AIDS. 2004;18:799–804. doi: 10.1097/00002030-200403260-00012. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SB, Gilbert RL, Brady AR, et al. CD4 Surveillance Scheme Advisory Group. CD4 cell counts in adults with newly diagnosed HIV infection: results of surveillance in England and Wales, 1990–1998. AIDS. 2000;14:853–861. doi: 10.1097/00002030-200005050-00012. [DOI] [PubMed] [Google Scholar]
- 29.Delmas M-C, Jadand C, DeVincenzi I, et al. SEROCO Study Group. Gender differences in CD4+ cell counts persist after HIV-1 infection. AIDS. 1997;11:1071–1073. [PubMed] [Google Scholar]
- 30.Hutchinson CM, Wilson C, Reichart CA, et al. CD4 lymphocyte concentrations in patients with newly identified HIV infection attending STD clinics. JAMA. 1991;266:253–256. [PubMed] [Google Scholar]
- 31.Luby S, Jones J, Horan J. Using CD4 counts to evaluate the stages of epidemiology of HIV infections in South Carolina Public Clinic Patients. Am J Public Health. 1994;84:377–381. doi: 10.2105/ajph.84.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Centers for Disease Control and Prevention, National Center for HIV, STD, and TB Prevention, Division of HIV/AIDS Prevention, Surveillance Branch. Diagnosis and reporting of HIV and AIDS in states with HIV/AIDS surveillance – United States, 1994–2000. MMWR Morb Mortal Wkly Rep. 2002;51:595–598. [Google Scholar]
- 33.CDC Collaborative Group. CD4 surveillance in Scotland: perspectives on severe HIV-related immunodeficiency. AIDS. 1997;11:1509–1517. [PubMed] [Google Scholar]
- 34.Biber CL, Jaker MA, Kloser P, et al. A study of sex differences in presentation for care of HIV. AIDS Patient Care STDs. 1999;13:103–110. doi: 10.1089/apc.1999.13.103. [DOI] [PubMed] [Google Scholar]
- 35.Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4+ T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002;185:1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 36.Maini MK, Gilson RJC, Chavda N, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med. 1996;72:27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Easterbrook PJ, Ye LM, Goetghebeur E, et al. Ten-year trends in CD4 cell counts at HIV and AIDS diagnosis in a London HIV clinic. AIDS. 2000;14:561–571. doi: 10.1097/00002030-200003310-00012. [DOI] [PubMed] [Google Scholar]
- 38.Swindells S, Cobos DF, Lee N, et al. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16:1832–1834. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 39.US Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States – 2002. HIV/AIDS Surveillance Report. 2002;14:1. Available at: http://www.cdc.gov/hiv/stats/hasrlink.htm Accessed February 3, 2005. [Google Scholar]
- 40.HIV/AIDS Surveillance Section, Communicable Diseases and Immunization Division, Michigan Department of Community Health. Quarterly HIV/AIDS analysis. 2002 Jan 1; Available at: http://www.michigan.gov/mdch Accessed February 3, 2005.
- 41.Inungu JN. An analysis of the serostatus approach to fighting the HIV epidemic. Am J Public Health. 2002;92:331. doi: 10.2105/ajph.92.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walensky RP, Losina E, Steger-Craven KA, et al. Identifying undiagnosed human immunodeficiency virus. Arch Intern Med. 2002;162:887–892. doi: 10.1001/archinte.162.8.887. [DOI] [PubMed] [Google Scholar]
- 43.Stanley B, Fraser J, Cox NH. Uptake of HIV screening in genitourinary medicine after change to “opt-out” consent. BMJ. 2003;326:1174. doi: 10.1136/bmj.326.7400.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]


