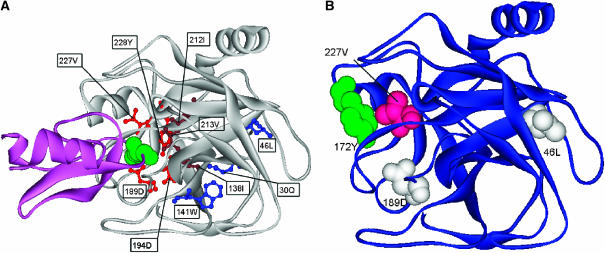
Figure 5.
(A) Structural mapping of CICD residues in the bovine beta-trypsin complex (gray) with pancreatic trypsin inhibitor (magenta). Residues belonging to the trypsin S1 pocket (red) are in contact with Lys15 of the pancreatic trypsin inhibitor (green). CICD residues (brown) located further from the binding site are likely to be important for the binding specificity, whereas those shown in blue reside in the core of the protein. (B) Correspondence between CICD residues and statistically coupled positions for trypsin, as detected by Ranganathan and co-workers. CICD residues (white) belong to the network of statistically coupled residues, whereas Val227 (pink) interacts with the statistically coupled residue Y172 (green).