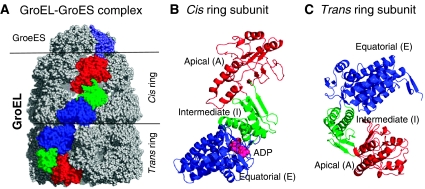
Figure 1.
Structure of the GroEL–GroES complex. (A) Space-filling model from the crystal structure determined by Xu et al (1997) (Protein Data Bank (PDB): 1AON). GroEL has a cylindrical structure, composed of two rings, termed the cis and trans rings, depending on the position of the GroES cap. Each ring in GroEL is composed of seven subunits. One subunit in each ring is shown in color (red, green and blue) in (A), along with one of the chains of the heptameric co-chaperonin (shown in gray/slate). The colored subunits on the GroES cap and the GroEL cis and trans rings correspond to three representative chains (identified as chains R, D and K in the PDB file (Berman et al, 2000)) whose communication dynamics will be examined below (see Figure 3C). (B) and (C) display ribbon diagrams of these two subunits belonging to the cis and trans rings, respectively. Each subunit consists of three domains, A, I and E, which refer to the apical, intermediate and equatorial domains, respectively. The corresponding residue ranges are: [A] Met193–Gly375; [I] Cys138–Gly192 (I1) and Val376–Gly410 (I2); and [E] Met1–Pro137 (E1) and Val411–Pro525 (E2). ADP molecule bound to the equatorial domain of the cis ring subunit is displayed in pink in panel B.