Abstract and Introduction
Abstract
Context
Bone mineral density (BMD) is used to diagnose osteoporosis, and often to measure efficacy in osteoporosis treatment trials; however, there is a poor correlation between lumbar spine BMD increases and vertebral fracture risk reduction in patients receiving treatment for osteoporosis.
Objective
The purpose of this article is to review the uses and limitations of BMD measurements and the relationship between BMD and bone strength.
Data Source/Study Selection
A MEDLINE literature search was conducted with the terms bone mineral density, fracture, osteoporosis, and bone strength as well as the generic names of osteoporosis therapies (alendronate, risedronate, raloxifene, teriparatide, and calcitonin). Search results were limited to English language journals and articles published within the last 20 years. Published abstracts from scientific meetings were also reviewed.
Conclusion
BMD measurement remains the most useful diagnostic tool for identifying patients with osteoporosis. Although they are helpful in guiding decisions to initiate osteoporosis treatment, subsequent changes in BMD provide an imperfect indicator of treatment efficacy. Analyses of clinical trials show an inconsistent relationship between increased spinal BMD and a decreased risk of vertebral fracture. Increased BMD accounts for less than 25% of the overall reduction in fracture risk in most instances. Consequently, fracture risk reduction itself remains the most clinically relevant therapeutic outcome of osteoporosis therapy.
Introduction
Bone mineral density (BMD) is widely used for diagnosing osteoporosis and determining the need for therapy in patients at risk, as well as for monitoring individual patients' responses to therapy. It is often used as a surrogate measure of efficacy in clinical trials of osteoporosis therapies. Although there is a consensus that osteoporosis trials with fracture incidence as a primary end point provide the most meaningful assessment of drug efficacy, these trials typically require large numbers of patients and often take at least 3 years to generate adequate data.[1] Because every patient has a BMD measurement but not every patient experiences a fracture, trials with BMD as a surrogate end point require fewer patients and can be completed in less time and at a lower cost than studies that assess fracture as an end point. However, the effect of antiresorptive drugs on BMD accounts for only a small portion of the overall reduction in fracture risk.[2,3] For example, the proportion of the reduction in vertebral fracture risk from the Fracture Intervention Trial (FIT) showed that improvement in spinal BMD explained only 16% of the risk reduction of vertebral fracture with alendronate therapy.[2]
This article reviews the uses and limitations of BMD measurements and the relationship between BMD and bone strength. A MEDLINE literature search was conducted with the terms bone mineral density, fracture, osteoporosis, and bone strength as well as the generic names of osteoporosis therapies (alendronate, risedronate, raloxifene, teriparatide, and calcitonin). Search results were limited to English language journals and articles published within the last 20 years. Published abstracts from scientific meetings were also searched to obtain more recent analyses on the relationship between BMD and fracture.
Epidemiology of Osteoporosis
Osteoporosis is a skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, resulting in decreased bone strength and predisposition to fractures.[4] This condition is a contributing factor in more than 1.5 million fractures per year in the United States, including an estimated 700,000 vertebral fractures, 300,000 hip fractures, 250,000 distal forearm fractures, and 250,000 fractures at other sites.[5] In the United States, an estimated 10 million people have osteoporosis and another 34 million have low bone mass (osteopenia), placing them at increased risk for this disorder.[6] According to the World Health Organization criteria, the 44 million people in the United States with either osteoporosis or low bone mass represent 55% of the population aged 50 years and older.[6] Primary osteoporosis can occur at any age but most commonly follows menopause in women; nearly 4 out of 5 individuals with osteoporosis in the United States are women.[6] Osteoporosis is often a silent disease of which the first clinical manifestation may be fracture. It is therefore frequently underrecognized and undertreated, even in older adults with a recent history of a low trauma fracture.[7]
Interpretation of BMD
Bone mineral content in adults at any time is dependent on peak bone density achieved during development and subsequent bone loss; therefore, low BMD can result from deficient bone accretion, accelerated bone loss, or both.[8] The diagnosis and classification of osteoporosis in postmenopausal women are based on BMD, which is reported as a T score and/or fracture history (Table 1).[9]
Table 1.
World Health Organization Criteria for Diagnosis of Osteoporosis Based on Bone Mineral Density
| Classification | Bone Mineral Density | T Score |
|---|---|---|
| Normal | Within 1 SD of reference mean* | −1 or above |
| Osteopenia | >1 but < 2.5 SD below reference mean | Below −1 but above −2.5 |
| Osteoporosis | 2.5 SD below reference mean | −2.5 or below |
| Severe osteoporosis | 2.5 SD below reference mean plus at least 1 fragility fracture | −2.5 or below |
Reference mean based on normal values for a young adult; SD = standard deviation
Data from: World Health Organ Tech Rep Ser. 1994;843:1–129
Bone densitometry is the gold standard for diagnosing osteoporosis, and dual-energy x-ray absorptiometry (DXA) is the preferred technology for measurement.[5,9,10] Because of its ease of use, low radiation exposure, and ability to measure BMD at both the hip and spine, DXA is the most commonly used technique to measure BMD.[11] Quantitative computed tomography (QCT) and peripheral measurements of BMD are useful for screening or assessing fracture risk but should not be used for diagnosis. Although QCT can distinguish between cortical and cancellous bone, its use as a screening and monitoring tool is limited by a lack of availability, relatively high radiation exposure, and higher cost.[11] DXA and QCT have been adapted for use at peripheral sites.[11] The following is a summary of techniques for quantitative measurement of BMD:
Central skeleton (spine and hip): DXA and QCT; and
Peripheral skeleton (wrist and heel): DXA, QCT, single-energy x-ray absorptiometry, radiographic absorptiometry, and quantitative ultrasound.
In untreated, older postmenopausal women, when the BMD is lower, the fracture risk is greater.[12] In general, fracture risk approximately doubles for each standard deviation below the mean young adult BMD (or for each −1 decrease in T score), regardless of fracture type and BMD measurement site.[13] Most BMD measurement sites have similar predictive ability for spine and hip fractures, with the exception of the spine and hip, which have better predictive ability for fractures, respectively.[13] However, it should be realized that there is a wide overlap in BMD scores of patients who do and do not sustain osteoporotic fractures. In a post hoc analysis of data from the Study of Osteoporotic Fractures, more than 50% of postmenopausal women who suffered from a hip fracture had T scores higher than −2.5.[14]
Another limitation of BMD is that the thresholds for intervention vary depending on the underlying condition. For example, patients with glucocorticoid-induced osteoporosis may develop fractures at BMD values higher than their postmenopausal counterparts.[15] Furthermore, although patients with type 2 diabetes have a higher mean BMD than age-matched controls, they are at a higher risk of nonvertebral fractures (hip, proximal humerus, and foot) but not vertebral fractures.[16]
The National Osteoporosis Foundation (NOF), the International Society for Clinical Densitometry (ISCD), the American College of Obstetricians and Gynecologists (ACOG), and the US Preventive Services Task Force (USPSTF) have published guidelines for the use of bone densitometry.[5,10,17,18] Postmenopausal women aged 65 years and older or postmenopausal women younger than 65 years with risk factors (eg, personal history of fracture as an adult, history of fragility fracture in a first-degree relative, low body weight, use of oral glucocorticoids for > 3 months, current smoking, and alcohol use) should receive a bone density test. In addition, those with fragility fractures should receive BMD testing because of their increased risk for future fractures. As discussed, those with medical conditions or those taking medications associated with bone loss (eg, glucocorticoids, anticonvulsants [phenytoin and phenobarbital], and cytotoxic drugs) are also at risk for osteoporosis and should be evaluated.
Although BMD measurements are useful for diagnosis, there is no precise and consistent relationship between a given increase in BMD and a specific decrease in fracture risk with osteoporosis therapy. This is not surprising because there are many contributors to bone strength apart from BMD (see next section). New technologies (eg, ultra-high-resolution peripheral QCT, 3-dimensional [3D] DXA, and 3D magnetic resonance imaging [MRI]) on the horizon that can noninvasively assess bone cross-sectional geometry and trabecular architecture may provide a more comprehensive picture of bone strength compared with 2-dimensional BMD measurements.
Bone Strength
The skeletal system contains 2 main types of tissue: porous cancellous (trabecular) bone and dense or compact cortical bone. Cancellous bone accounts for approximately 15% of total bone mass. The vertebrae and pelvic bones contain relatively high amounts of cancellous tissue and are common sites of osteoporotic fractures, whereas the long bones (eg, femoral neck) contain a relatively high amount of cortical bone.[19]
Cancellous bone contains a complex network of trabeculae. Aging bone is characterized by loss and/or thinning of trabeculae, and these structural changes are more pronounced in individuals with osteoporosis.[20] Alterations in the 3D microarchitecture of bone tissue compromise its structural integrity and strength.[21] Even small changes that do not substantially affect overall bone mass can have a marked impact on structural competence.
Although it has been shown that about 70% of the variation in compressive strength of bone is determined by its mineral density,[22] prediction of trabecular bone strength may be greatly improved by including measures of bone quality. BMD changes do not reflect the effects of drug therapy on other factors that affect bone strength. In addition to BMD, bone strength and susceptibility to fracture depend on trabecular connectivity and arrangement, biomechanical properties (such as elasticity, strain/stress response, and failure point), and other factors, such as bone size, shape, turnover, and architecture.[22] For example, a recent analysis of 693 postmenopausal women from the Vertebral Efficacy With Risedronate Therapy (VERT) studies showed that changes in bone turnover markers at 3–6 months predicted about one half of vertebral fracture reduction with risedronate after 1 year and about two thirds of the reduction after 3 years.[23]
BMD vs Fracture Prevention
In the absence of an accurate single measure of overall bone strength, BMD is the most common tool used to assess fracture risk in the clinic. Although there is a definite relationship between the change in BMD and reduction in fracture risk, the magnitude of the relationship is controversial. This suggests that measurement of BMD alone cannot account for all of the treatment-related effects on other important contributors to bone strength and fracture risk reduction. This point is supported in the following review of data from clinical studies and meta-analyses.
Clinical Trials
The association between increases in BMD resulting from treatment and reductions in fracture risk is illustrated by a review of clinical data from major clinical trials of antiresorptive osteoporosis treatments in postmenopausal women. In the risedronate VERT studies, the risk of new morphometric vertebral fractures was significantly (both P ≤ .001) reduced within 1 year by 65%65%[24] and 61%[25] in risedronate 5-mg/day recipients, and the risk of clinical vertebral fractures was reduced within 6 months.[26] Furthermore, in a post hoc pooled analysis of 1172 postmenopausal women with osteoporosis, with or without a prevalent vertebral fracture, risedronate 5 mg/day reduced the risk of nonvertebral fractures (ie, fractures of the clavicle, humerus, wrist, pelvis, hip, or leg) significantly as early as 6 months (P = .048) after therapy initiation.[27] In the FIT studies, a post hoc analysis of postmenopausal women with confirmed osteoporosis found that alendronate reduced 1-year clinical vertebral fracture risk by 59% (P = .030).[28] These early fracture risk reductions observed before maximal changes in spine BMD were achieved suggest that bisphosphonates increase bone strength through factors that may be independent of BMD.
In clinical trials, both raloxifene[29] and salmon calcitonin[30] produced modest but significant improvements in BMD and significantly decreased vertebral fracture risk after 3 and 5 years of treatment, respectively, although neither drug significantly reduced the risk of nonvertebral fracture in prospective analyses. In neither case did the change in BMD fully predict the reduction in risk of vertebral fracture (Table 2).
Table 2.
Relative Contribution of Spine BMD Improvement to Reduction in Vertebral Fracture Risk From Trials of Antiresorptive Drugs
| Drug | Contribution of Spine BMD Improvement to Reduction in Vertebral Fracture Risk |
|---|---|
| Alendronate | 16%[2] |
| 17%[41] | |
| 40% (maximum 67%)[42] | |
| Calcitonin | ≤ 4%[42] |
| Estradiol | ≤ 43%[42] |
| Raloxifene | 4%[39] |
| Risedronate | 18%[3] |
| 28%[43] |
BMD = bone mineral density
BMD increases do not always result in favorable fracture outcomes. In a 2-year study in 354 women with postmenopausal osteoporosis and vertebral fracture at baseline, the improvement in spinal BMD was significantly greater in patients who received a fluoride salt along with calcium and vitamin D than in those who received only calcium and vitamin D (10.8% vs 2.4%, respectively; P = .0001); however, there were no significant between-group differences in the incidence rates of new vertebral or hip fractures.[31] Therefore, despite large gains in BMD, there was no fracture reduction observed with fluoride therapy, indicating that factors in addition to bone density (such as bone quality) play important roles in bone strength and fracture risk reduction. Of note, these results and those from other studies have raised concerns over the quality of new bone synthesized following fluoride therapy.[31,32]
Exogenous parathyroid hormone (1–34), or teriparatide, is an anabolic agent that stimulates bone formation. Once-daily injections of teriparatide 20 or 40 mcg/day for a median duration of 21 months significantly increased spinal BMD over placebo in a nearly dose-dependent manner (by 8.6% and 12.6%, respectively; both P < .001), but reductions in the risk of vertebral fracture were similar for both dosage groups (65% and 69%, respectively; both P ≤ .001)[33] and consistent with reported antifracture efficacy of the bisphosphonates. If fracture risk reduction was proportional to gains in BMD, one would have expected greater reductions in fracture risk with teriparatide therapy than those that were observed.
Drug-related changes in bone quality may play an important role in improving bone strength. In an analysis of paired bone biopsy samples (baseline and posttreatment), teriparatide increased cortical thickness and 3D trabecular connectivity.[34] In a paired biopsy study, risedronate was shown to preserve microarchitecture (trabecular number, thickness, and separation).[35] Alendronate treatment decreased cortical porosity and increased uniformity of mineralization compared with placebo.[36]
Meta-analyses
Attempts to clarify the apparent discrepancy between a change in BMD and a change in fracture risk have yielded conflicting results. In a meta-analysis of antiresorptive therapies that used Poisson regression analysis to estimate the contribution of BMD change to the reduction in incident vertebral fractures, a 54% fracture risk reduction was estimated for an 8% gain in spinal BMD.[37] However, this analysis also showed that at the intercept (no change in BMD), there was an approximate 20% reduction in vertebral fracture incidence, suggesting that factors other than changes in BMD contribute to risk reduction (Figure).
Figure.
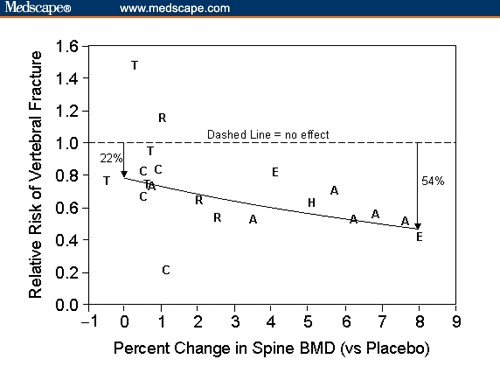
Relative risk of new vertebral fracture vs change in spine BMD relative to placebo from clinical trials of postmenopausal osteoporosis. The solid line represents the Poisson regression results (A = alendronate; BMD = bone mineral density; C = calcitonin; E = etidronate; H = hormone replacement (estrogen); R = raloxifene; T = tiludronate). Reprinted with permission from: Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85:231–236. Copyright 2000, The Endocrine Society.)
A more recent meta-analysis that also used Poisson regression showed that both BMD gain and bone turnover marker reduction were able to explain the reduction in nonvertebral fracture risk.[38] These studies demonstrated a relationship between increases in BMD and reduction in fracture risk; however, the changes in BMD alone could not account for the magnitude of the risk reduction.
A more recent meta-analysis that also used Poisson regression showed that both BMD gain and bone turnover marker reduction were able to explain the reduction in nonvertebral fracture risk.[38] These studies demonstrated a relationship between increases in BMD and reduction in fracture risk; however, the changes in BMD alone could not account for the magnitude of the risk reduction.
Analyses of FIT and data from other trials showed that reported improvements in spine BMD accounted for variable and limited percentages of the observed effects on fracture risk (Table 2). An analysis of FIT data with logistic models of individual patient data reported that improvements in spinal BMD produced by alendronate accounted for 16% of the achieved reduction in the risk of vertebral fracture.[2] With logistic regression models, the percentage change in lumbar spine or femoral neck BMD with raloxifene (60− and 120-mg/day pooled dose groups) similarly did not predict the extent to which vertebral fracture risk decreased after 3 years of treatment.[39]
An analysis of the VERT data indicated that an increase in lumbar spine BMD with risedronate 5 mg/day was associated with reduced risk of vertebral fracture.[3] However, for large increases in spinal BMD (between 5% and 9%), there was little gain in fracture risk reduction. The Cox proportional hazards model predicted an 18% reduction in the risk of vertebral fracture, whereas the actual 3-year risk reductions in VERT-North American[24] and VERT-Multinational[25] were 41% and 49%, respectively. In a separate analysis of the combined VERT data, BMD increases in the spine and hip over 3 years accounted for 12.2% and 5.5%, respectively, of the proportion of nonvertebral fracture risk reduction, suggesting that the relationship between BMD increases and nonvertebral fracture risk reduction may be nonlinear.[40]
Conclusion
BMD measurement remains the most useful clinical tool for identifying patients with osteoporosis. Several organizations, such as the ACOG, ISCD, NOF, and the USPSTF, have published guidelines for BMD testing. Although they are useful in guiding decisions to initiate treatment, subsequent changes in BMD do not fully explain reductions in fracture risk. Therefore, in evaluating the efficacy of treatments in osteoporosis clinical trials, fracture end points are the most relevant, and caution should be used when consulting BMD data only to interpret clinical efficacy.
In clinical practice, serial BMD measures have some clinical utility in monitoring response to therapy, but it is important to keep in mind that fracture-protection benefit may be realized before BMD gains are detected. If there is no change in BMD, it does not mean that therapy is not working, because treatment may have prevented bone loss. Gains in BMD reflect an important aspect of bone strength, and along with bone turnover, they may provide positive feedback about the treatment regimen to the patient. The ISCD recommends that the time between BMD follow-up testing should be individualized.[10] The typical follow-up schedule is 1 year after initiation of new therapy and longer intervals thereafter.
Fracture risk depends on a number of variables that contribute to bone strength in addition to BMD, such as bone size, shape, architecture, and turnover. Some osteoporosis therapies have been shown to improve or preserve qualities that contribute to bone strength, which can help account for the reductions in fracture risk observed with these agents separate from changes in BMD. In the clinical practice setting, however, there is no practical method to measure overall bone quality and resistance to fracture in individual patients. Newer, noninvasive techniques may provide some hope of an improved surrogate marker for therapy in the future. In the meantime, fracture risk reduction remains the most clinically relevant therapeutic outcome of osteoporosis therapy.
References
- 1.Division of Metabolic and Endocrine Drug Products. Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Available at: http://www.fda.gov/cder/guidance/osteo.pdf Accessed April 24, 2005.
- 2.Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 3.Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261. doi: 10.1385/jcd:7:3:255. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 5.National Osteoporosis Foundation. Physician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2003. [Google Scholar]
- 6.National Osteoporosis Foundation. America's Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 7.Cuddihy MT, Gabriel SE, Crowson CS, et al. Osteoporosis intervention following distal forearm fractures: a missed opportunity? Arch Intern Med. 2002;162:421–426. doi: 10.1001/archinte.162.4.421. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JS. Osteoporosis. In: Cecil RL, Goldman L, Bennett JC, editors. Cecil Textbook of Medicine. 21st ed. Philadelphia, Pa: WB Saunders Co.; 2000. pp. 1367–1373. [Google Scholar]
- 9.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 10.Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the international society for clinical densitometry. J Clin Densitom. 2004;7:1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 11.Miller PD, Zapalowski C, Kulak CA, Bilezikian JP. Bone densitometry: the best way to detect osteoporosis and to monitor therapy. J Clin Endocrinol Metab. 1999;84:1867–1871. doi: 10.1210/jcem.84.6.5710. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 13.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright S, Phipps K, Stone J, et al. A large proportion of fractures in postmenopausal women occur with baseline bone mineral density T-score −2.5. J Bone Miner Res. 2001;16:S155. [Google Scholar]
- 15.Luengo M, Picado C, Del Rio L, et al. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax. 1991;46:803–806. doi: 10.1136/thx.46.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 17.American College of Obstetricians and Gynecologists, Women's Health Care Physicians. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 50, January 2003. Obstet Gynecol. 2004;103:203–216. [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526–528. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]
- 19.Mundy GR. Bone remodeling. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. pp. 30–38. [Google Scholar]
- 20.Parfitt AM, Mathews CH, Villanueva AR, et al. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 22.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(suppl3):S13–S18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 23.Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 24.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 25.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 26.Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within 6 months. Curr Med Res Opin. 2004;20:433–439. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- 27.Harrington JT, Ste-Marie LG, Brandi ML, et al. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 28.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 30.Chesnut CH, III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 31.Meunier PJ, Sebert JL, Reginster JY, et al. Fluoride salts are no better at preventing new vertebral fractures than calcium-vitamin D in postmenopausal osteoporosis: the FAVO Study. Osteoporos Int. 1998;8:4–12. doi: 10.1007/s001980050041. [DOI] [PubMed] [Google Scholar]
- 32.Pak CY, Zerwekh JE, Antich P. Anabolic effects of fluoride on bone. Trends Endocrinol Metab. 1995;6:229–234. doi: 10.1016/1043-2760(95)00111-t. [DOI] [PubMed] [Google Scholar]
- 33.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 34.Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 35.Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–432. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- 36.Roschger P, Rinnerthaler S, Yates J, et al. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 37.Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85:231–236. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- 38.Hochberg MC, Greenspan S, Wasnich RD, et al. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87:1586–1592. doi: 10.1210/jcem.87.4.8415. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar S, Mitlak BH, Wong M, et al. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Watts NB, Felsenberg D, Johnson TD, Li Z, Eastell R. Small proportion of nonvertebral fracture risk reduction explained by BMD increases [abstract P2-487]. Paper presented at: The Endocrine Society's 85th Annual Meeting; June 19–22, 2003; Philadelphia, Pennsylvania. [Google Scholar]
- 41.Cummings S, Black D, Pearson J, et al. How much of the reduction in risk of vertebral fractures by alendronate is explained by increased spine BMD? [Abstract] J Bone Miner Res. 1999;14:S159. [Google Scholar]
- 42.Cummings SR, Black DM, Vogt TM FIT Research Group. Changes in BMD substantially underestimate the anti-fracture effects of alendronate and other antiresorptive drugs [abstract 29] J Bone Miner Res. 1996;11:S102. [Google Scholar]
- 43.Li Z, Meredith M, Hoseyni MS. A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med. 2001;20:3175–3188. doi: 10.1002/sim.984. [DOI] [PubMed] [Google Scholar]


