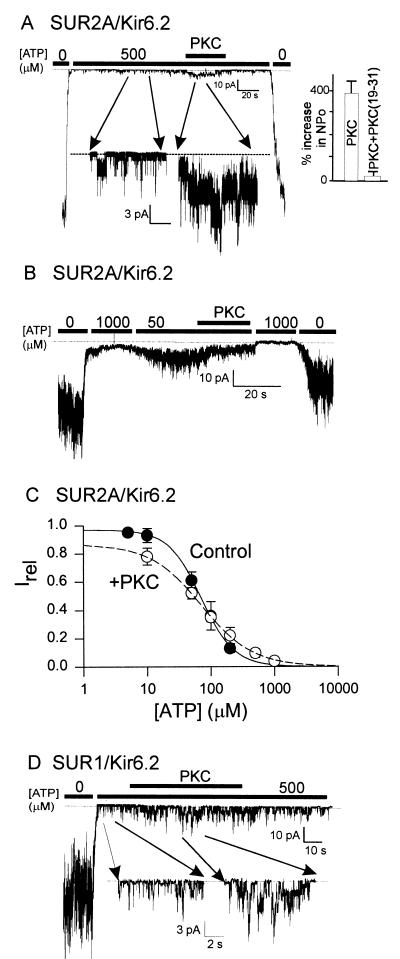
Figure 1.
PKC and mechanisms of action on the KATP channel. (A and B) Representative currents from an inside-out patch containing wild-type (WT) (SUR2A/Kir6.2) cardiac KATP channels. The patches were excised and held at −50 mV in symmetrical K+ (140 mM) and then exposed to different internal ATP concentrations. The addition of constitutively active PKC (20 nM) is indicated by the solid bar. (A) Note that PKC activates SUR2A/Kir6.2 channel activity in the presence of 500 μM ATP. Inset shows cumulative data from five patches (PKC) or three patches [PKC(19–31)]. The PKC inhibitor peptide PKC(19–31) was used at a concentration of 5 μM. (B) Note that PKC inhibits SUR2A/Kir6.2 channel activity at low (50-μM) levels of ATP. (C) Estimates of IC50 were obtained by grouping data from between four and seven patches, at each ATP concentration, in the absence (●) or presence (○) of PKC. Data were fitted to the equation Irel = 1/{1 + ([ATP]/IC50)n}, where Irel is the current relative to the maximal current observed in the absence of ATP and n is the Hill coefficient. IC50 values of 70 μM and 71 μM were determined in the absence and presence of PKC, respectively. (D) Representative WT (SUR1/Kir6.2) pancreatic beta cell KATP channel current response to PKC from an inside-out patch under the same conditions as in A.