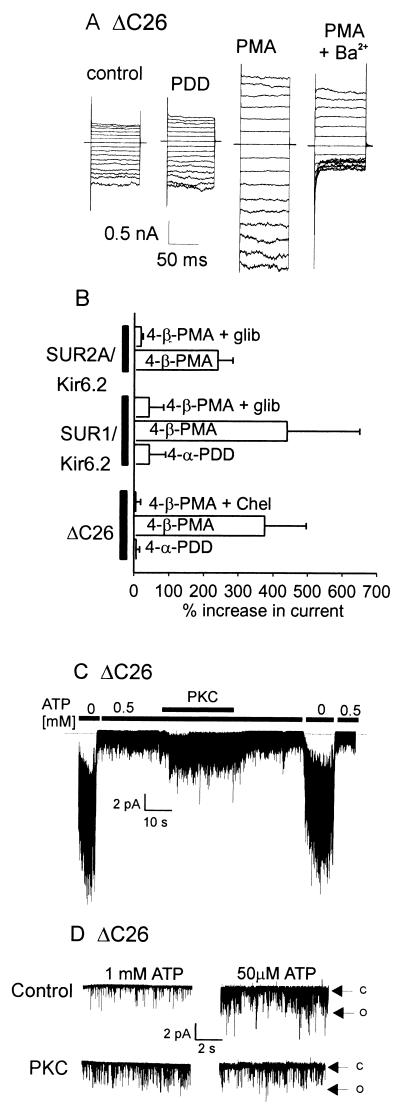
Figure 2.
PKC activates KATP channels in the presence and absence of the SUR subunit. (A) Representative whole-cell current recordings from a tsA201 cell expressing the Kir6.2 ΔC26 truncation mutant by using the nystatin-perforated patch technique (20). Recordings were made 6 min after the application of either the inactive phorbol analog (PDD, 100 nM) or the active phorbol ester PMA (100 nM). Block by external barium (2 mM) was used to indicate KATP current amplitude. (B) Cumulative data from whole-cell current experiments using ΔC26 alone, SUR1/Kir6.2, or SUR2A/Kir6.2 (n = 4–8 cells for each group). (C) Recording from an inside-out patch containing multiple ΔC26 channels that shows an increase in activity on application of constitutively active PKC (20 nM). (D) Inside-out patch recordings from ΔC26 channels showing the effects of constitutively active PKC on channel activity at an internal [ATP] of either 1 mM or 50 μM. Note that PKC activates ΔC26 in the presence of high (1 mM) ATP but inhibits ΔC26 activity at low (50 μM) ATP. Holding potential in both C and D was −50 mV using symmetrical, 140 mM K+. C and O indicate closed and open levels, respectively.