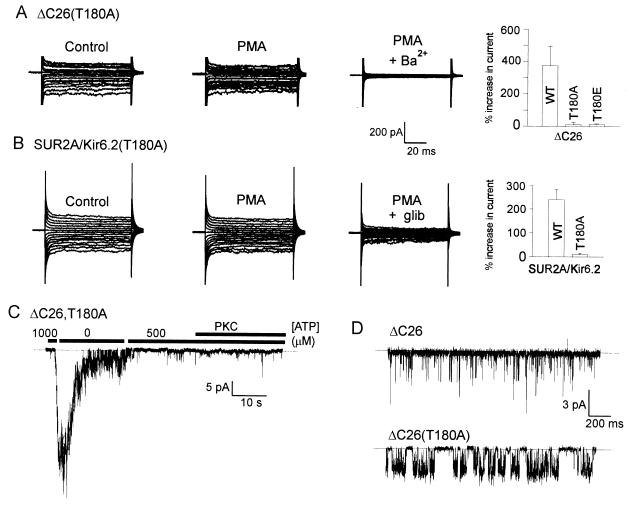
Figure 4.
Phorbol ester (PMA) does not increase currents in cells expressing KATP channels with the T180A Kir6.2 mutation. (A) Representative whole-cell current recordings of ΔC26(T180A) channel activity in response to 100 nM PMA (6 min) by using the nystatin-perforated patch technique. (B) Whole-cell perforated patch current recording from a cell coexpressing SUR2A and a full length Kir6.2(T180A) mutant showing the effect of 100 nM PMA (6 min). The histograms represent cumulative data from between four and six cells; wild-type (WT) data are replotted from Fig. 2B for comparison. (C) Representative inside-out patch recording of ΔC26(T180A) channel activity showing the response to ATP and constitutively active PKC. No effect of PKC on ΔC26(T180A) channels was observed in any of three patches tested. (D) Representative inside-out patch, single-channel recordings from ΔC26 and ΔC26(T180A) channels. Longer open times than for the wild-type ΔC26 were observed in each of three patches containing the ΔC26(T180A) mutant channel. Recordings were made in the absence of ATP at a holding potential of −50 mV in symmetrical, 140 mM K+.