Abstract
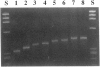
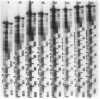
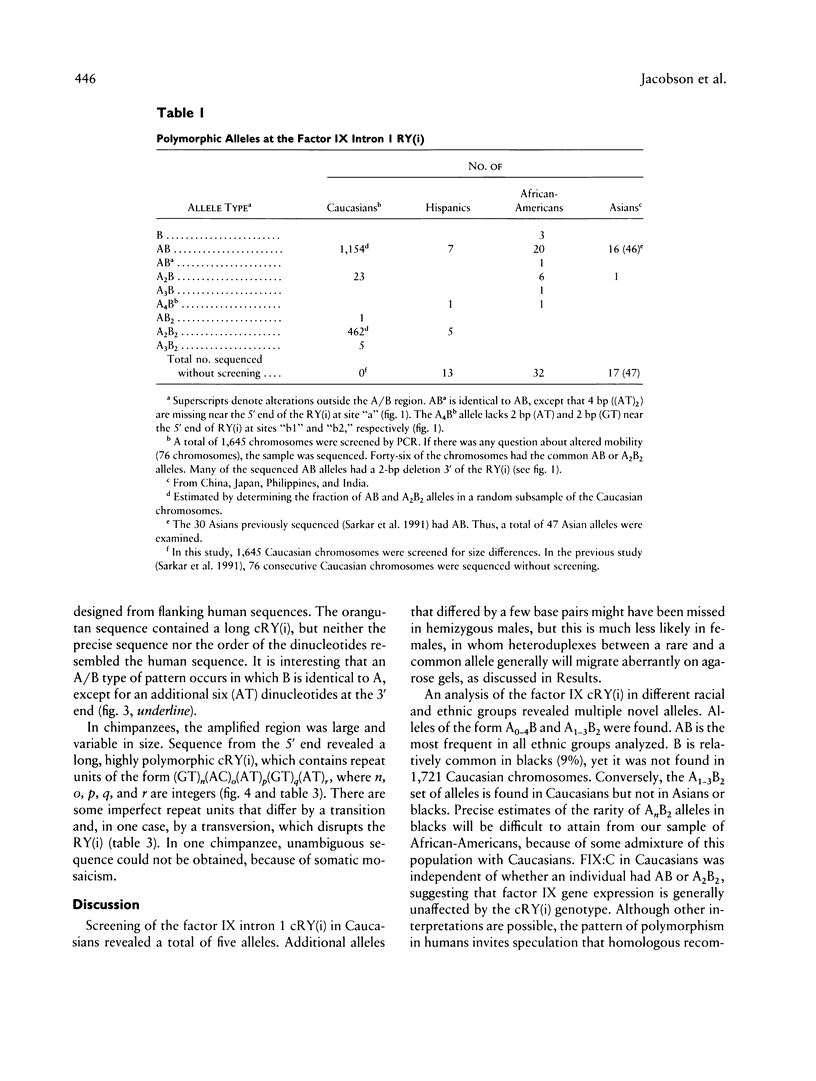
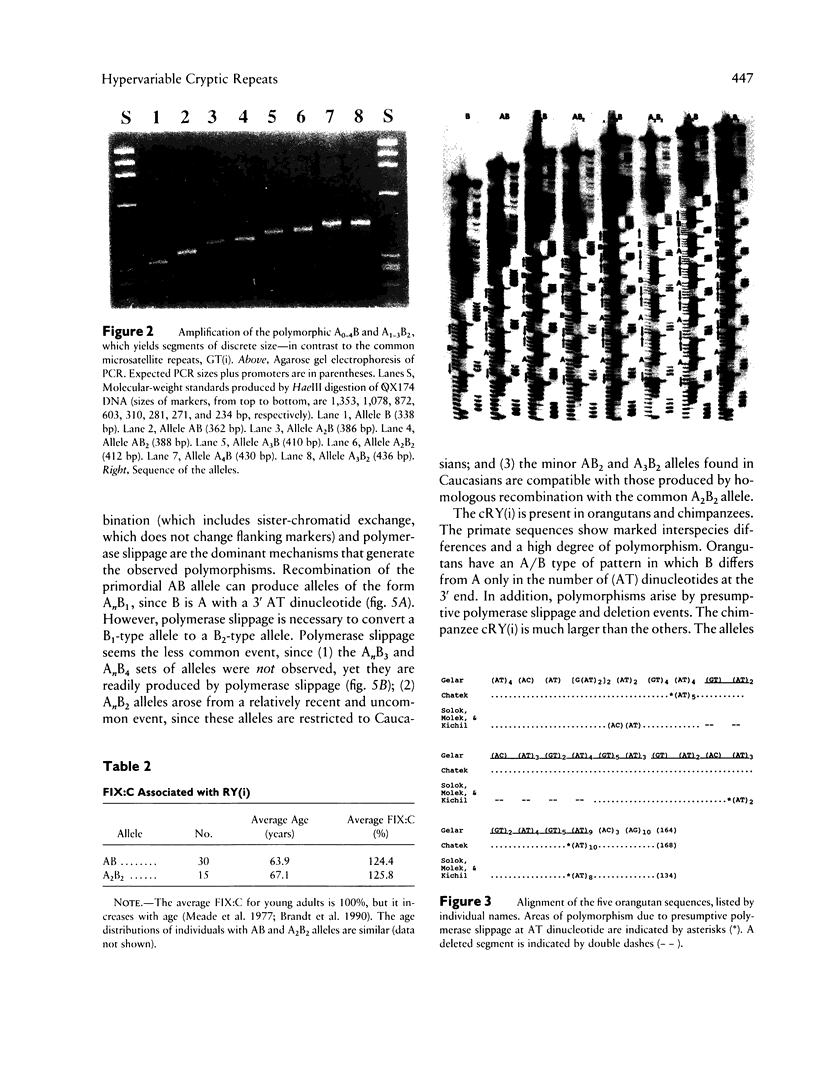
Alternating purine and pyrimidine repeats (RY(i)) are an abundant source of polymorphism. The subset with long tandem repeats of GT or AC (GT(i)) have been studied extensively, but cryptic RY(i) (i.e., no single tandem repeat predominates) have received little attention. The factor IX gene has a polymorphic cryptic RY(i) of 142-216 bp. Previously, there were four known polymorphic alleles, of the form AB, A2B, A2B2, and A3B2, where A = (GT)(AC)3(AT)3(GT)(AT)4 and B = A with an additional 3' AT dinucleotide. To further characterize this locus, we examined more than 1,700 additional human chromosomes and determined the sequences of the homologous sites in orangutans and chimpanzees. The novel alleles found in humans expand the repertoire of A/B alleles to A0-4B1 and A1-3B2. The AnB2 series are abundant in Caucasians but are absent in blacks and Asians. Conversely, the A0B1 allele is common in blacks but is not found in more than 1,700 Caucasian chromosomes. The data are compatible with a model in which recombination is more frequent than polymerase slippage at this locus. In orangutans, the RY(i) is present, but the sequence is markedly different. An A/B-type of pattern was discerned in which B differs from A by an additional six (AT) dinucleotides at the 3' end. In chimpanzees, the size of the RY(i) locus was greatly expanded, and the sequence showed a novel pattern of hypervariability in which there are many tandem repeats of the form (GT)n(AC)o(AT)p(GT)q(AT)s, where n, o, p, q, and s are different integers. The sequences of the factor IX intron 1 cryptic RY(i) in three primates provide perspective on the range of possible patterns of polymorphism. Analysis of the patterns suggests how the RY(i) can be conserved during evolution, while the precise sequence varies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya A., Lilley D. M. The contrasting structures of mismatched DNA sequences containing looped-out bases (bulges) and multiple mismatches (bubbles). Nucleic Acids Res. 1989 Sep 12;17(17):6821–6840. doi: 10.1093/nar/17.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A., Murchie A. I., Lilley D. M. RNA bulges and the helical periodicity of double-stranded RNA. Nature. 1990 Feb 1;343(6257):484–487. doi: 10.1038/343484a0. [DOI] [PubMed] [Google Scholar]
- Blaho J. A., Wells R. D. Left-handed Z-DNA and genetic recombination. Prog Nucleic Acid Res Mol Biol. 1989;37:107–126. doi: 10.1016/s0079-6603(08)60696-0. [DOI] [PubMed] [Google Scholar]
- Bottema C. D., Koeberl D. D., Ketterling R. P., Bowie E. J., Taylor S. A., Lillicrap D., Shapiro A., Gilchrist G., Sommer S. S. A past mutation at isoleucine 397 is now a common cause of moderate/mild haemophilia B. Br J Haematol. 1990 Jun;75(2):212–216. doi: 10.1111/j.1365-2141.1990.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Brandt J. T., Arkin C. F., Bovill E. G., Rock W. A., Triplett D. A. Evaluation of APTT reagent sensitivity to factor IX and factor IX assay performance. Results from the College of American Pathologists Survey Program. Arch Pathol Lab Med. 1990 Feb;114(2):135–141. [PubMed] [Google Scholar]
- Caskey C. T., Pizzuti A., Fu Y. H., Fenwick R. G., Jr, Nelson D. L. Triplet repeat mutations in human disease. Science. 1992 May 8;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Hauge X. Y., Grandy D. K., Eubanks J. H., Evans G. A., Civelli O., Litt M. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991 Jul;10(3):527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- Hsieh C. H., Griffith J. D. Deletions of bases in one strand of duplex DNA, in contrast to single-base mismatches, produce highly kinked molecules: possible relevance to the folding of single-stranded nucleic acids. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4833–4837. doi: 10.1073/pnas.86.13.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Sarkar G., Ketterling R. P., Chen S. H., Sommer S. S. Recurrent nonsense mutations at arginine residues cause severe hemophilia B in unrelated hemophiliacs. Hum Genet. 1990 Apr;84(5):387–390. doi: 10.1007/BF00195805. [DOI] [PubMed] [Google Scholar]
- Luty J. A., Guo Z., Willard H. F., Ledbetter D. H., Ledbetter S., Litt M. Five polymorphic microsatellite VNTRs on the human X chromosome. Am J Hum Genet. 1990 Apr;46(4):776–783. [PMC free article] [PubMed] [Google Scholar]
- Meade T. W., North W. R., Chakrabarti R., Haines A. P., Stirling Y. Population-based distributions of haemostatic variables. Br Med Bull. 1977 Sep;33(3):283–288. doi: 10.1093/oxfordjournals.bmb.a071448. [DOI] [PubMed] [Google Scholar]
- Morral N., Nunes V., Casals T., Estivill X. CA/GT microsatellite alleles within the cystic fibrosis transmembrane conductance regulator (CFTR) gene are not generated by unequal crossingover. Genomics. 1991 Jul;10(3):692–698. doi: 10.1016/0888-7543(91)90454-m. [DOI] [PubMed] [Google Scholar]
- Naylor L. H., Clark E. M. d(TG)n.d(CA)n sequences upstream of the rat prolactin gene form Z-DNA and inhibit gene transcription. Nucleic Acids Res. 1990 Mar 25;18(6):1595–1601. doi: 10.1093/nar/18.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Paynton C., Sommer S. S. Segments containing alternating purine and pyrimidine dinucleotides: patterns of polymorphism in humans and prevalence throughout phylogeny. Nucleic Acids Res. 1991 Feb 11;19(3):631–636. doi: 10.1093/nar/19.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treco D., Arnheim N. The evolutionarily conserved repetitive sequence d(TG.AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis. Mol Cell Biol. 1986 Nov;6(11):3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. The Z-DNA motif d(TG)30 promotes reception of information during gene conversion events while stimulating homologous recombination in human cells in culture. Mol Cell Biol. 1990 Feb;10(2):785–793. doi: 10.1128/mcb.10.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L. Informativeness of human (dC-dA)n.(dG-dT)n polymorphisms. Genomics. 1990 Aug;7(4):524–530. doi: 10.1016/0888-7543(90)90195-z. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Winship P. R., Anson D. S., Rizza C. R., Brownlee G. G. Carrier detection in haemophilia B using two further intragenic restriction fragment length polymorphisms. Nucleic Acids Res. 1984 Dec 11;12(23):8861–8872. doi: 10.1093/nar/12.23.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. Transcription is associated with Z-DNA formation in metabolically active permeabilized mammalian cell nuclei. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2259–2263. doi: 10.1073/pnas.88.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]