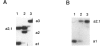Abstract
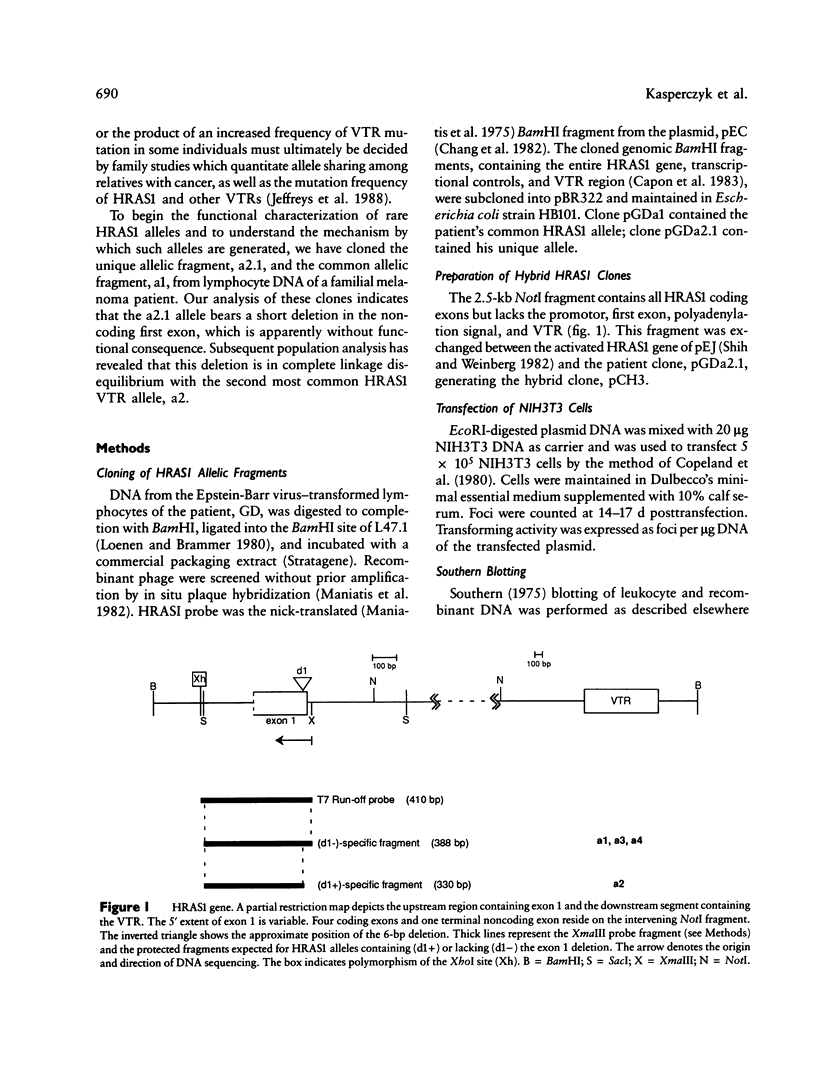
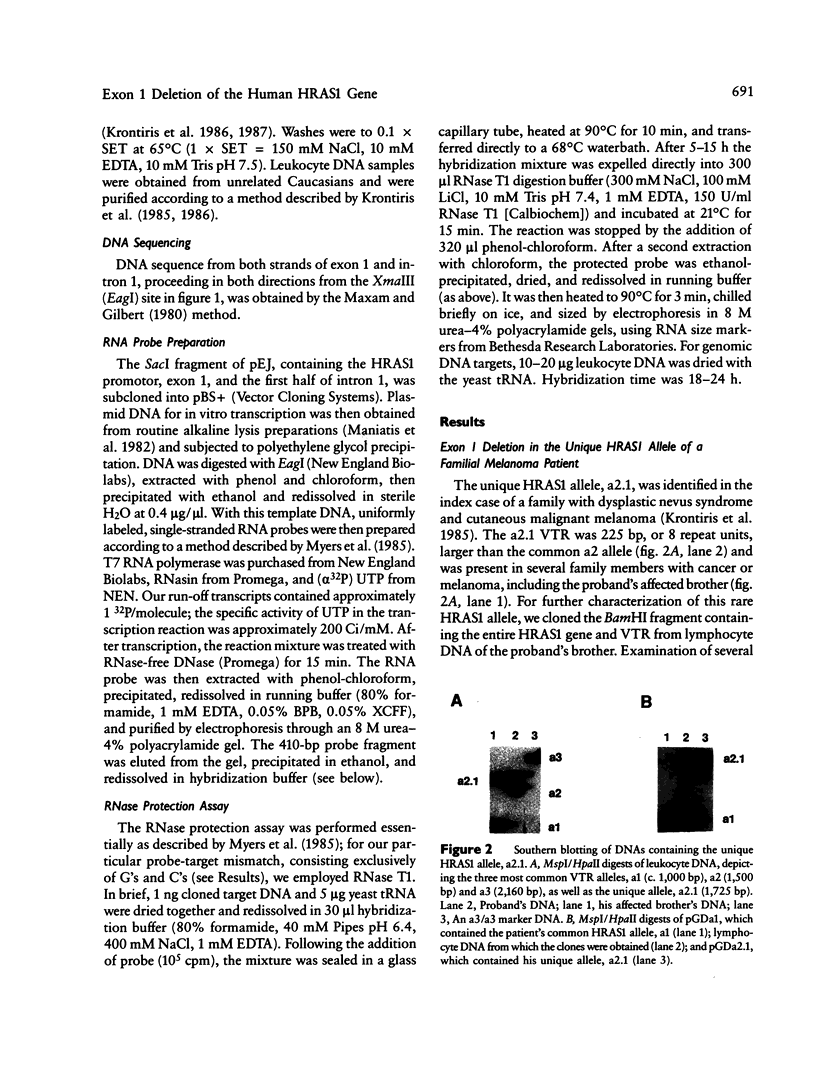
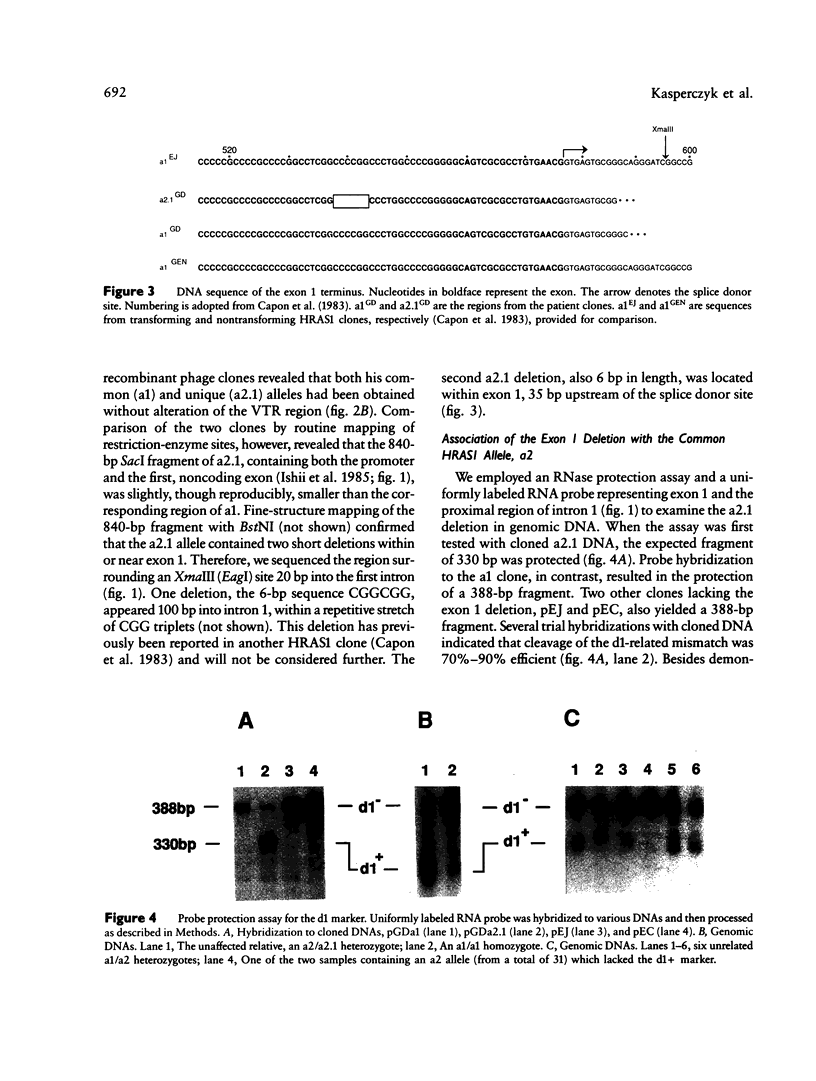
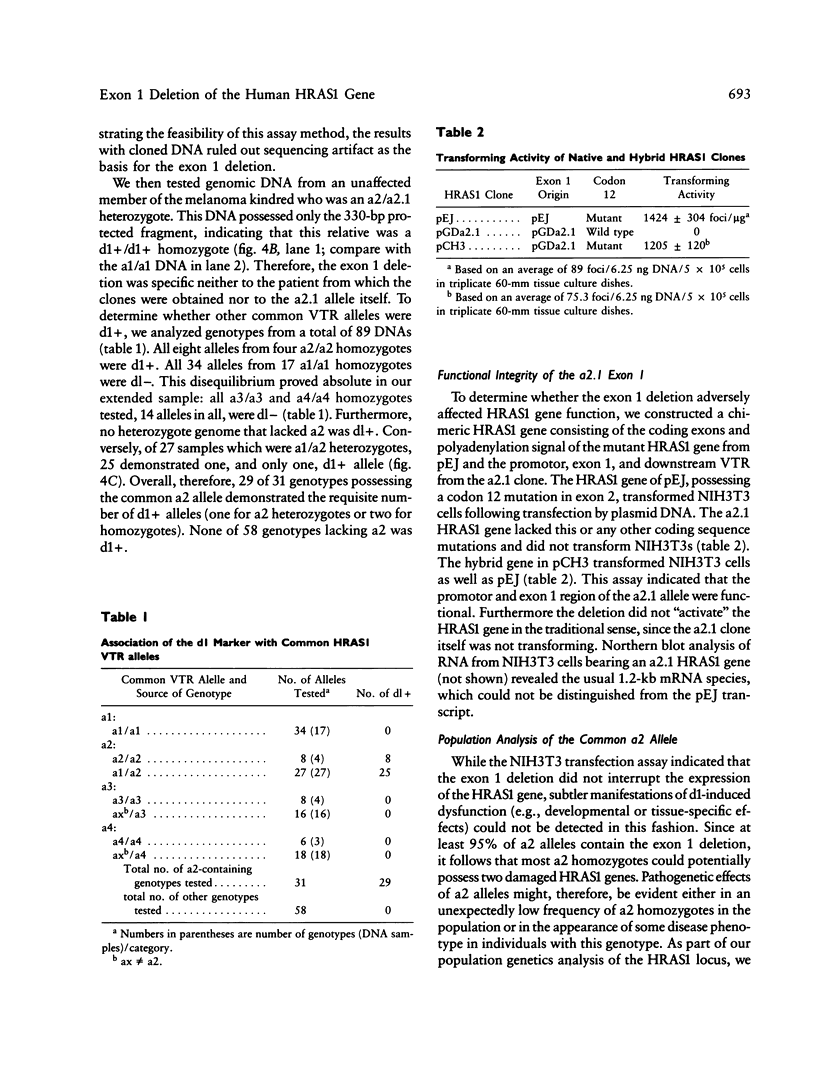
We have detected a 6-bp deletion in the untranslated first exon of a unique HRAS1 gene cloned from lymphocyte DNA of a familial melanoma patient. The deletion is without apparent functional consequence. Using an RNase protection assay, we have demonstrated the deletion in leukocyte DNAs of individuals unrelated to the patient. In these cases, the deletion marker is specifically associated with one class of common HRAS1 allele, thereby establishing the origin of the unique allele. We discuss the means by which DNA sequence heterogeneity at other loci may be rapidly analyzed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Boehm T. L., Hirth H. P., Kornhuber B., Drahovsky D. Oncogene amplifications, rearrangements, and restriction fragment length polymorphisms in human leukaemia. Eur J Cancer Clin Oncol. 1987 Jun;23(6):623–629. doi: 10.1016/0277-5379(87)90257-4. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Carter G., Worwood M., Jacobs A. The Ha-ras polymorphism in myelodysplasia and acute myeloid leukaemia. Leuk Res. 1988;12(5):385–391. doi: 10.1016/0145-2126(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Ceccherini-Nelli L., De Re V., Viel A., Molaro G., Zilli L., Clemente C., Boiocchi M. Ha-ras-1 restriction fragment length polymorphism and susceptibility to colon adenocarcinoma. Br J Cancer. 1987 Jul;56(1):1–5. doi: 10.1038/bjc.1987.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler L. A., Ghazi H., Jones P. A., Boukamp P., Fusenig N. E. Allele-specific methylation of the human c-Ha-ras-1 gene. Cell. 1987 Aug 28;50(5):711–717. doi: 10.1016/0092-8674(87)90329-1. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Copper G. M. Transformation by subgenomic fragments of Rous sarcoma virus DNA. Cell. 1980 Apr;19(4):863–870. doi: 10.1016/0092-8674(80)90077-x. [DOI] [PubMed] [Google Scholar]
- Corell B., Zoll B. Comparison between the allelic frequency distribution of the Ha-ras 1 locus in normal individuals and patients with lymphoma, breast, and ovarian cancer. Hum Genet. 1988 Jul;79(3):255–259. doi: 10.1007/BF00366247. [DOI] [PubMed] [Google Scholar]
- Gerhard D. S., Dracopoli N. C., Bale S. J., Houghton A. N., Watkins P., Payne C. E., Greene M. H., Housman D. E. Evidence against Ha-ras-1 involvement in sporadic and familial melanoma. Nature. 1987 Jan 1;325(6099):73–75. doi: 10.1038/325073a0. [DOI] [PubMed] [Google Scholar]
- Hayward N. K., Keegan R., Nancarrow D. J., Little M. H., Smith P. J., Gardiner R. A., Seymour G. J., Kidson C., Lavin M. F. c-Ha-ras-1 alleles in bladder cancer, Wilms' tumour and malignant melanoma. Hum Genet. 1988 Feb;78(2):115–120. doi: 10.1007/BF00278178. [DOI] [PubMed] [Google Scholar]
- Heighway J., Thatcher N., Cerny T., Hasleton P. S. Genetic predisposition to human lung cancer. Br J Cancer. 1986 Apr;53(4):453–457. doi: 10.1038/bjc.1986.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Merlino G. T., Pastan I. Promoter region of the human Harvey ras proto-oncogene: similarity to the EGF receptor proto-oncogene promoter. Science. 1985 Dec 20;230(4732):1378–1381. doi: 10.1126/science.2999983. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Colb M., Mitcheson H. D., Parkinson D. R. Human restriction fragment length polymorphisms and cancer risk assessment. J Cell Biochem. 1986;30(4):319–329. doi: 10.1002/jcb.240300405. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Colb M., Parkinson D. R. Unique allelic restriction fragments of the human Ha-ras locus in leukocyte and tumour DNAs of cancer patients. 1985 Jan 31-Feb 6Nature. 313(6001):369–374. doi: 10.1038/313369a0. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Mitcheson H. D., Lonergan J. A., Begg C., Parkinson D. R. Human hypervariable sequences in risk assessment: rare Ha-ras alleles in cancer patients. Environ Health Perspect. 1987 Dec;76:147–153. doi: 10.1289/ehp.8776147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lidereau R., Escot C., Theillet C., Champeme M. H., Brunet M., Gest J., Callahan R. High frequency of rare alleles of the human c-Ha-ras-1 proto-oncogene in breast cancer patients. J Natl Cancer Inst. 1986 Sep;77(3):697–701. doi: 10.1093/jnci/77.3.697. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Radice P., Pierotti M. A., Borrello M. G., Illeni M. T., Rovini D., Della Porta G. HRAS1 proto-oncogene polymorphisms in human malignant melanoma: TaqI defined alleles significantly associated with the disease. Oncogene. 1987;2(1):91–95. [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Holmes L. Transcriptional enhancer activity in the variable tandem repeat DNA sequence downstream of the human Ha-ras 1 gene. FEBS Lett. 1987 Jun 22;218(1):41–46. doi: 10.1016/0014-5793(87)81014-1. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Shaw H. M., Roberts C., Grace J., Stewart M. M., McCarthy W. H., Kefford R. F. Harvey-ras oncogene restriction fragment alleles in familial melanoma kindreds. Br J Cancer. 1986 Nov;54(5):787–790. doi: 10.1038/bjc.1986.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Oscier D. G., Flint J., Wainscoat J. S. Ha-ras hypervariable alleles in myelodysplasia. Nature. 1986 May 1;321(6065):84–85. doi: 10.1038/321084a0. [DOI] [PubMed] [Google Scholar]
- Thompson T. C., Southgate J., Kitchener G., Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989 Mar 24;56(6):917–930. doi: 10.1016/0092-8674(89)90625-9. [DOI] [PubMed] [Google Scholar]
- Wyllie F. S., Wynford-Thomas V., Lemoine N. R., Williams G. T., Williams E. D., Wynford-Thomas D. Ha-ras restriction fragment length polymorphisms in colorectal cancer. Br J Cancer. 1988 Feb;57(2):135–138. doi: 10.1038/bjc.1988.28. [DOI] [PMC free article] [PubMed] [Google Scholar]