Abstract
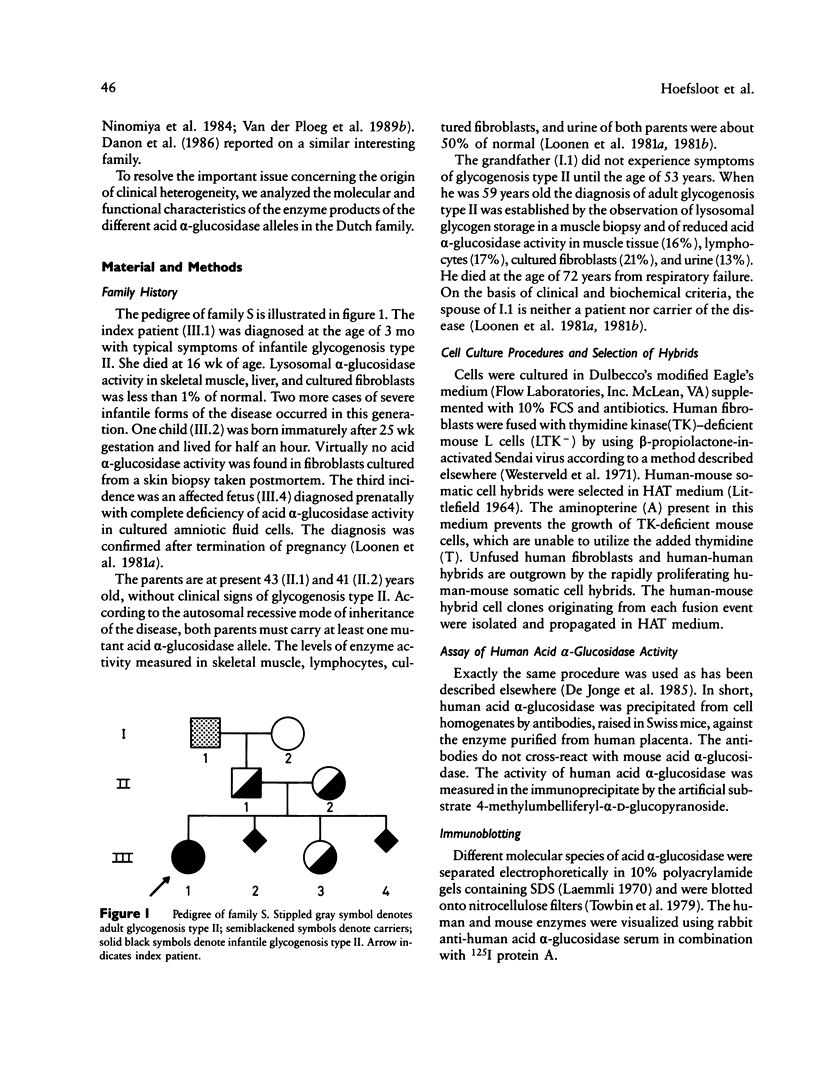
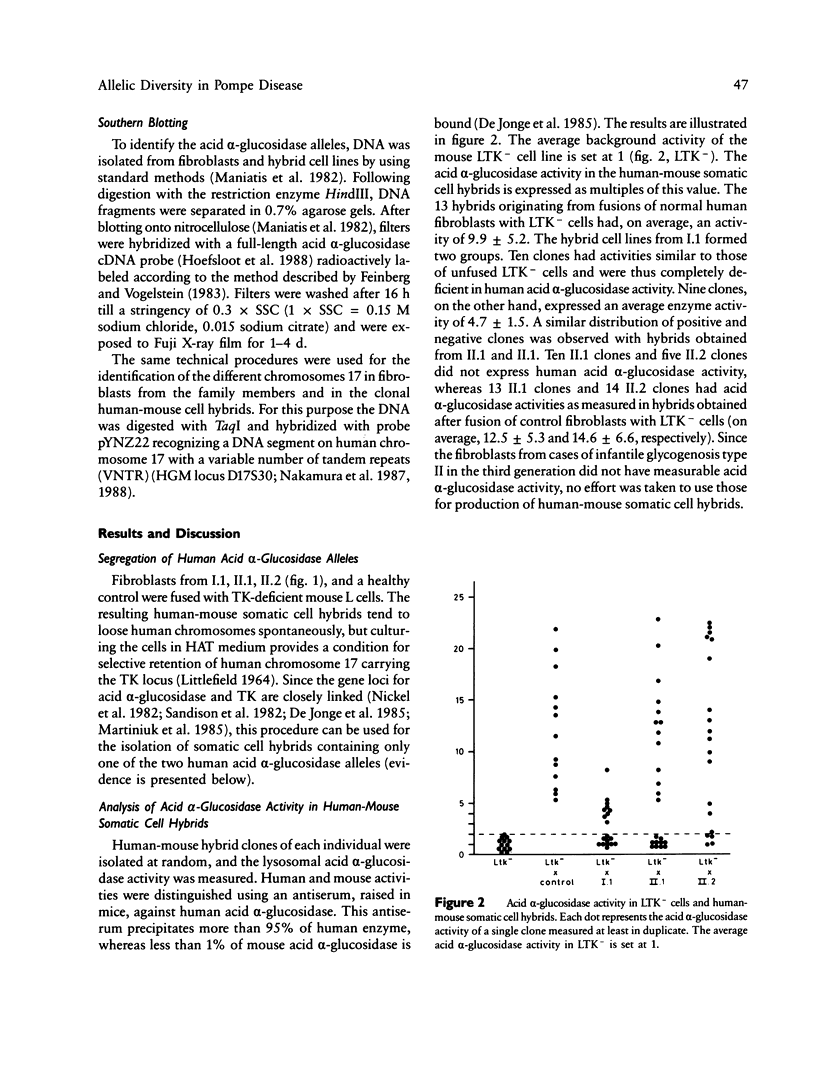
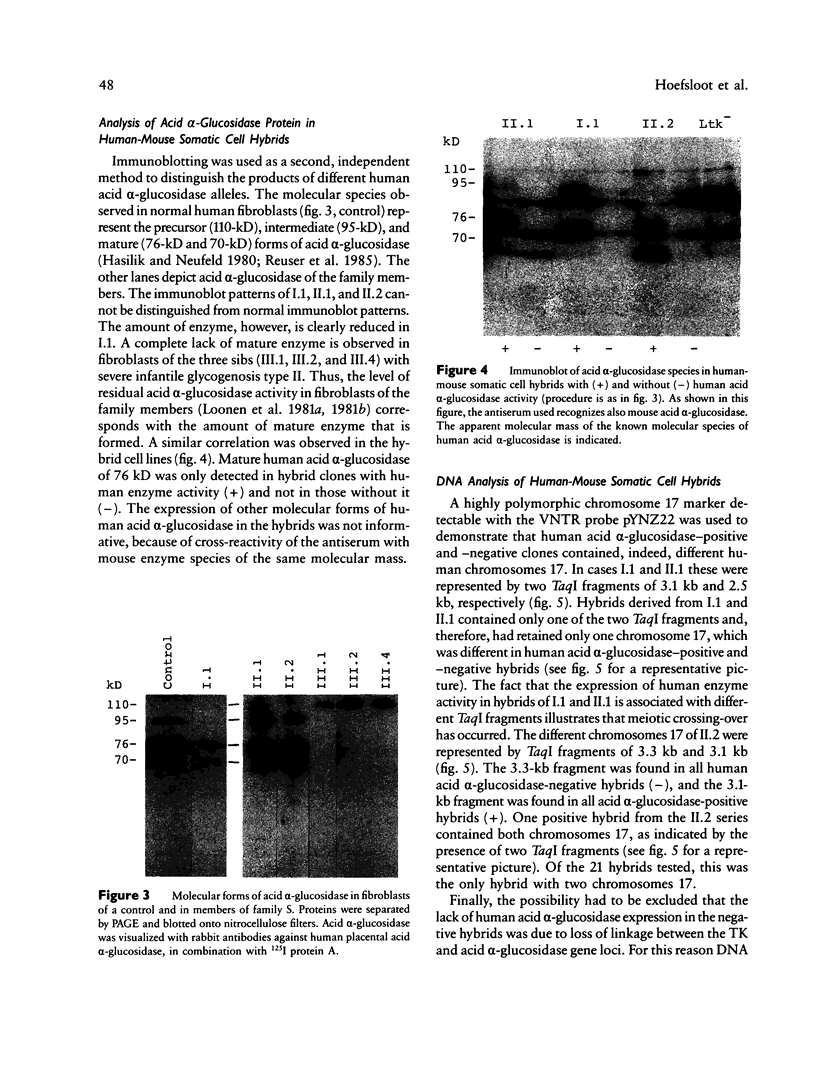
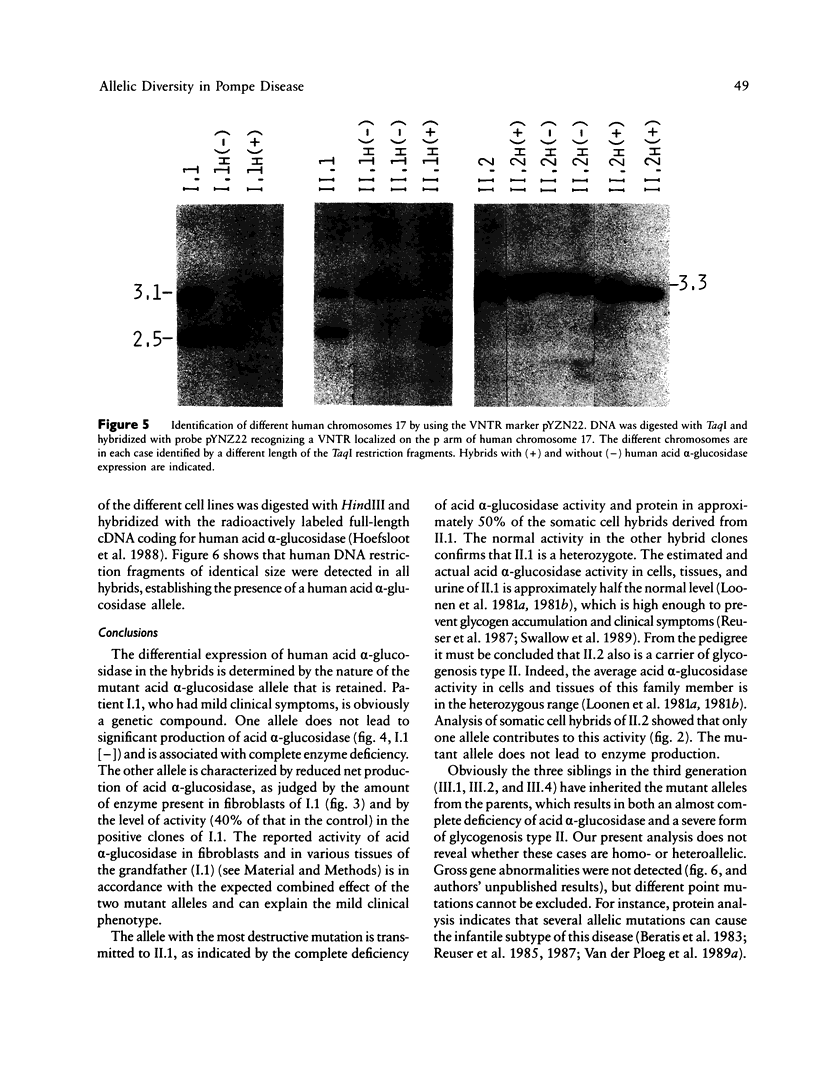
To define the cause of clinical heterogeneity in glycogenosis type II we have studied the inheritance and molecular nature of acid alpha-glucosidase deficiency in a rare family with severe infantile as well as mild late-onset variants of this disease. The (mutant) acid alpha-glucosidase alleles of crucial family members were segregated in human-mouse somatic cell hybrids to investigate their individual function. Two types of mutant alleles were identified. The first leads to complete deficiency of acid alpha-glucosidase. Homozygosity of this allele is demonstrated in three cases of severe infantile glycogenosis type II in the family under study. The second mutant allele is characterized by a reduced net production of catalytically active acid alpha-glucosidase, resulting in partial enzyme deficiency. The eldest patient in the family, with very mild clinical symptoms, is shown to be a compound heterozygote having both types of mutant alleles. These studies emphasize the effect of allelic diversity on the level of residual acid alpha-glucosidase activity and on the clinical course of glycogenosis type II.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini C., Engel A. G. Comparative study of acid maltase deficiency. Biochemical differences between infantile, childhood, and adult types. Arch Neurol. 1972 Apr;26(4):344–349. doi: 10.1001/archneur.1972.00490100074007. [DOI] [PubMed] [Google Scholar]
- Arpaia E., Dumbrille-Ross A., Maler T., Neote K., Tropak M., Troxel C., Stirling J. L., Pitts J. S., Bapat B., Lamhonwah A. M. Identification of an altered splice site in Ashkenazi Tay-Sachs disease. Nature. 1988 May 5;333(6168):85–86. doi: 10.1038/333085a0. [DOI] [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Acid alpha-glucosidase: kinetic and immunologic properties of enzyme variants in health and disease. Isozymes Curr Top Biol Med Res. 1983;11:25–36. [PubMed] [Google Scholar]
- Busch H. F., Koster J. F., van Weerden T. W. Infantile and adult-onset acid maltase deficiency occurring in the same family. Neurology. 1979 Mar;29(3):415–416. doi: 10.1212/wnl.29.3.415. [DOI] [PubMed] [Google Scholar]
- Danon M. J., DiMauro S., Shanske S., Archer F. L., Miranda A. F. Juvenile-onset acid maltase deficiency with unusual familial features. Neurology. 1986 Jun;36(6):818–822. doi: 10.1212/wnl.36.6.818. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J. F., Busch H. F., Slee R. G., Van Weerden T. W. Glycogenosis type II: the infantile- and late-onset acid maltase deficiency observed in one family. Clin Chim Acta. 1978 Aug 1;87(3):451–453. doi: 10.1016/0009-8981(78)90191-2. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loonen M. C., Schram A. W., Koster J. F., Niermeijer M. F., Busch H. F., Martin J. J., Brouwer-Kelder B., Mekes W., Slee R. G., Tager J. M. Identification of heterozygotes for glycogenosis 2 (acid maltase deficiency). Clin Genet. 1981 Jan;19(1):55–63. doi: 10.1111/j.1399-0004.1981.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Ellenbogen A., Hirschhorn K., Hirschhorn R. Further regional localization of the genes for human acid alpha glucosidase (GAA), peptidase D (PEPD), and alpha mannosidase B (MANB) by somatic cell hybridization. Hum Genet. 1985;69(2):109–111. doi: 10.1007/BF00293278. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Hirschhorn R. Characterization of neutral isozymes of human alpha-glucosidase: differences in substrate specificity, molecular weight and electrophoretic mobility. Biochim Biophys Acta. 1981 Apr 14;658(2):248–261. doi: 10.1016/0005-2744(81)90295-3. [DOI] [PubMed] [Google Scholar]
- Mehler M., DiMauro S. Residual acid maltase activity in late-onset acid maltase deficiency. Neurology. 1977 Feb;27(2):178–184. doi: 10.1212/wnl.27.2.178. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Hogikyan N. D. Different mutations in Ashkenazi Jewish and non-Jewish French Canadians with Tay-Sachs disease. Science. 1986 Jun 27;232(4758):1646–1648. doi: 10.1126/science.3754980. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Ballard L., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pYNZ22) on chromosome 17p [D17S30]. Nucleic Acids Res. 1988 Jun 24;16(12):5707–5707. doi: 10.1093/nar/16.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Ninomiya N., Matsuda I., Matsuoka T., Iwamasa T., Nonaka I. Demonstration of acid alpha-glucosidase in different types of Pompe disease by use of an immunochemical method. J Neurol Sci. 1984 Nov-Dec;66(2-3):129–139. doi: 10.1016/0022-510x(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Koster J. F., Hoogeveen A., Galjaard H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am J Hum Genet. 1978 Mar;30(2):132–143. [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Willemsen R., Swallow D., Tager J. M., Galjaard H. Clinical diversity in glycogenosis type II. Biosynthesis and in situ localization of acid alpha-glucosidase in mutant fibroblasts. J Clin Invest. 1987 Jun;79(6):1689–1699. doi: 10.1172/JCI113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandison A., Broadhead D. M., Bain A. D. Elucidation of an unbalanced chromosome translocation by gene dosage studies. Clin Genet. 1982 Jul;22(1):30–36. doi: 10.1111/j.1399-0004.1982.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Kroos M., Van der Ploeg A. T., Griffiths B., Islam I., Marenah C. B., Reuser A. J. An investigation of the properties and possible clinical significance of the lysosomal alpha-glucosidase GAA*2 allele. Ann Hum Genet. 1989 May;53(Pt 2):177–184. doi: 10.1111/j.1469-1809.1989.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg A. T., Hoefsloot L. H., Hoogeveen-Westerveld M., Petersen E. M., Reuser A. J. Glycogenosis type II: protein and DNA analysis in five South African families from various ethnic origins. Am J Hum Genet. 1989 Jun;44(6):787–793. [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg A. T., Kroos M. A., Swallow D. M., Reuser A. J. An investigation of the possible influence of neutral alpha-glucosidases on the clinical heterogeneity of glycogenosis type II. Ann Hum Genet. 1989 May;53(Pt 2):185–192. doi: 10.1111/j.1469-1809.1989.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Visser R. P., Meera Khan P., Bootsma D. Loss of human genetic markers in man--Chinese hamster somatic cell hybrids. Nat New Biol. 1971 Nov 3;234(44):20–24. doi: 10.1038/newbio234020a0. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., de Smit S., Kroos M. A., Reuser A. J. Cotransfer of syntenic human genes into mouse cells using isolated metaphase chromosomes or cellular DNA. Hum Genet. 1985;69(1):32–38. doi: 10.1007/BF00295526. [DOI] [PubMed] [Google Scholar]
- van der Ploeg A. T., Bolhuis P. A., Wolterman R. A., Visser J. W., Loonen M. C., Busch H. F., Reuser A. J. Prospect for enzyme therapy in glycogenosis II variants: a study on cultured muscle cells. J Neurol. 1988 Sep;235(7):392–396. doi: 10.1007/BF00314479. [DOI] [PubMed] [Google Scholar]