Abstract
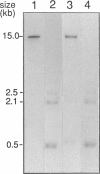
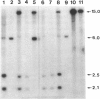
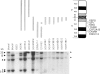
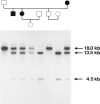
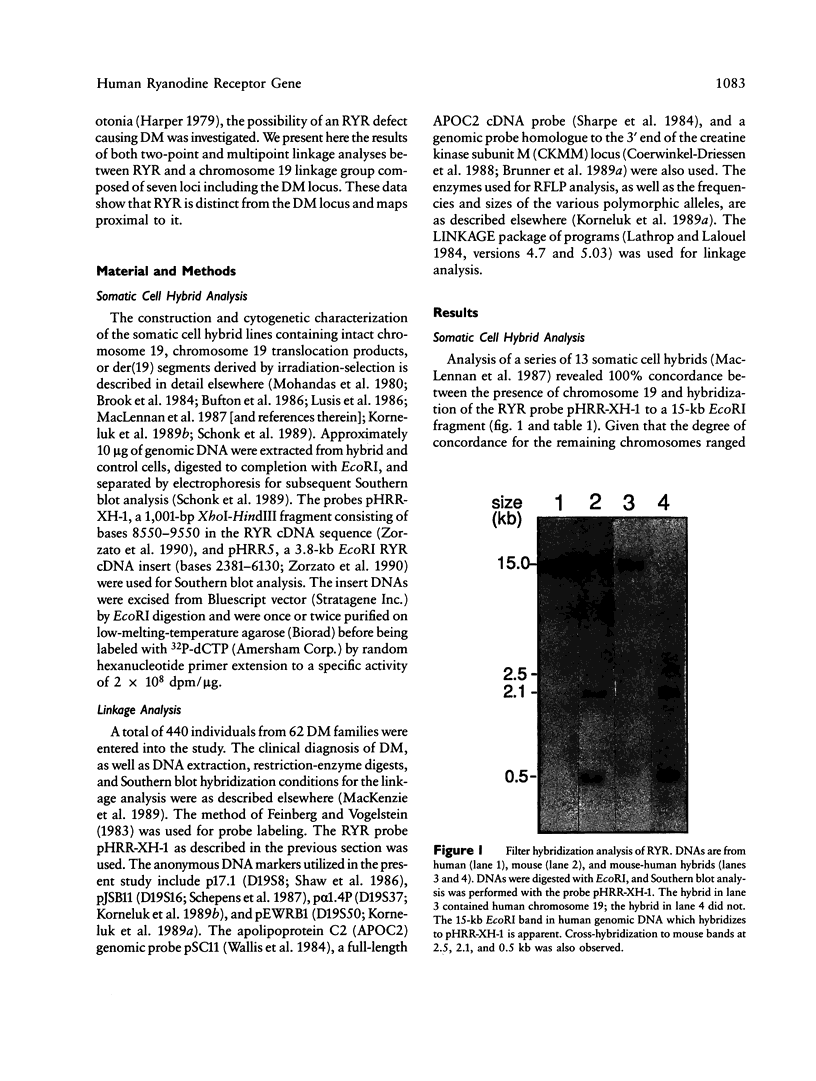
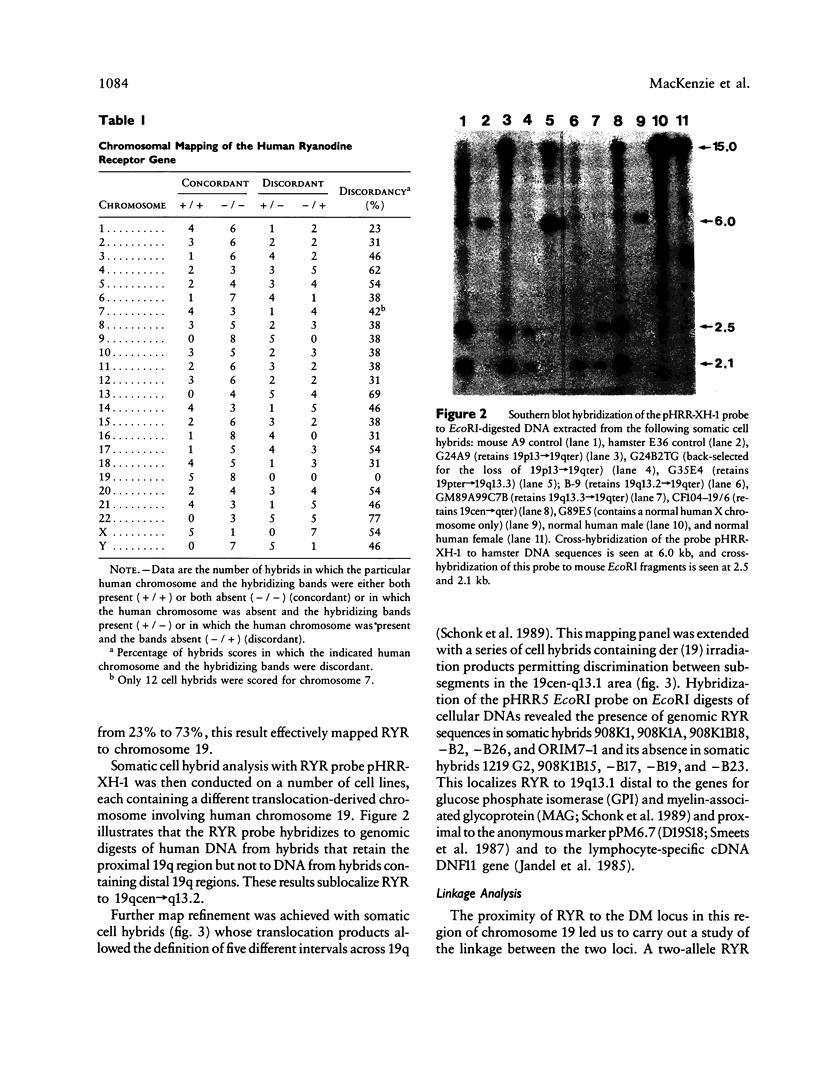
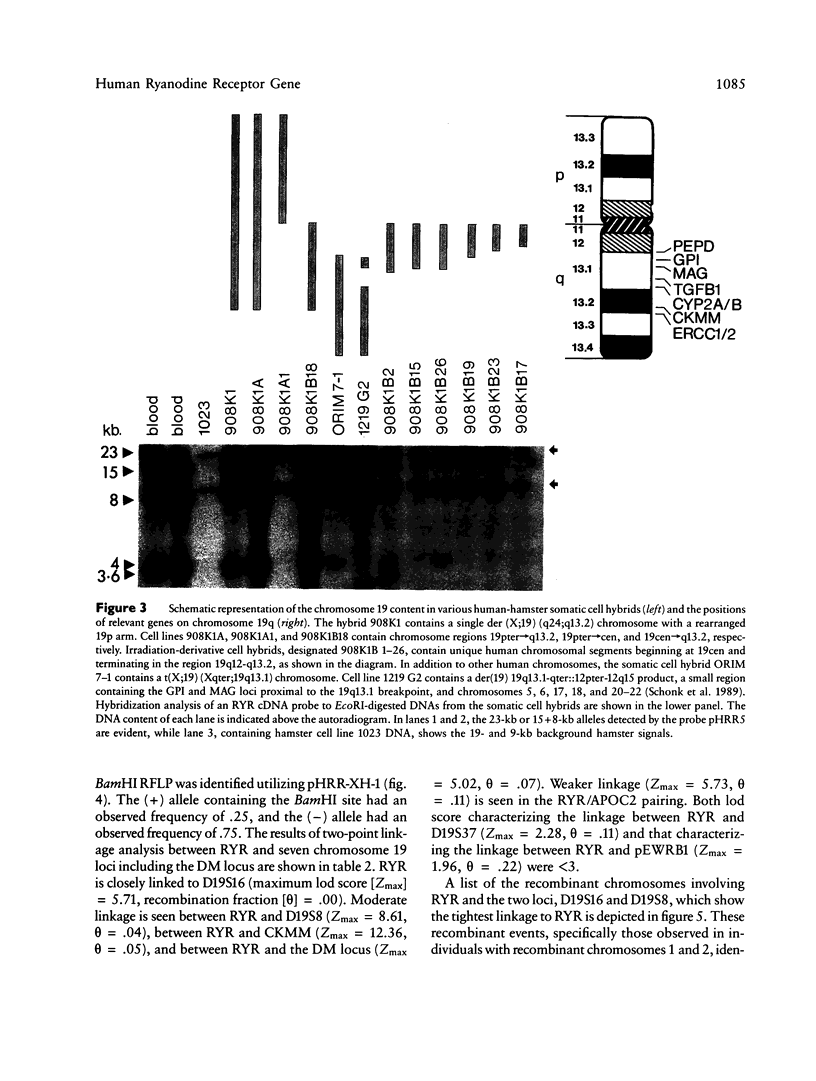
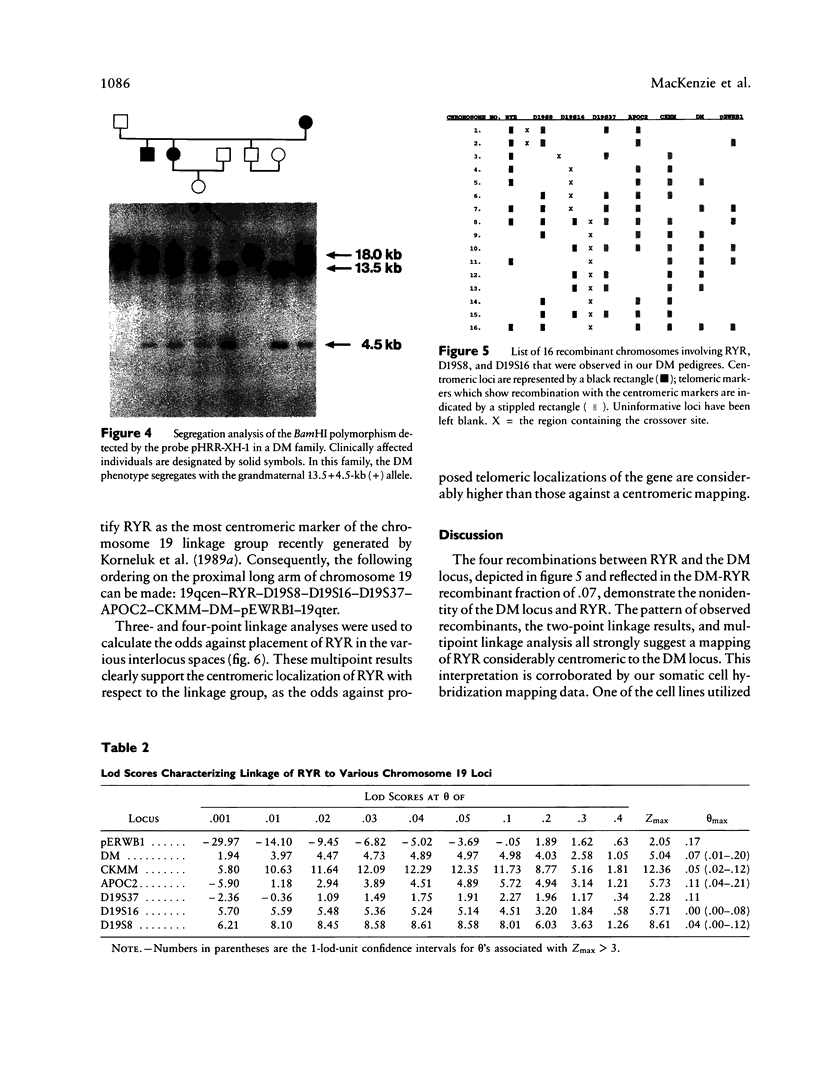
The recent cloning of cDNA encoding the Ca++ release channel (ryanodine receptor) of human sarcoplasmic reticulum has enabled us to use somatic cell hybrids to localize the ryanodine receptor gene (RYR) to the proximal long arm of human chromosome 19. Studies with additional hybrids containing deletions or translocations in chromosome 19 enabled us to localize RYR to 19q13.1 in a region distal to GPI/MAG and proximal to D19S18/DNF11. On the basis that the myotonic dystrophy (DM) locus maps near this region and that myotonia could result from a defect in the ryanodine receptor, we examined the linkage between the DM locus and RYR. Our results, showing several DM-RYR recombinants, rule out an RYR defect as the cause of DM. However, localization of RYR to a region of human chromosome 19 which is syntenic to an area of pig chromosome 6 containing the HAL gene responsible for porcine malignant hyperthermia supports the candidacy of RYR for this disorder.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britt B. A. Etiology and pathophysiology of malignant hyperthermia. Fed Proc. 1979 Jan;38(1):44–48. [PubMed] [Google Scholar]
- Brook J. D., Shaw D. J., Meredith L., Bruns G. A., Harper P. S. Localisation of genetic markers and orientation of the linkage group on chromosome 19. Hum Genet. 1984;68(4):282–285. doi: 10.1007/BF00292584. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Korneluk R. G., Coerwinkel-Driessen M., MacKenzie A., Smeets H., Lambermon H. M., van Oost B. A., Wieringa B., Ropers H. H. Myotonic dystrophy is closely linked to the gene for muscle-type creatine kinase (CKMM). Hum Genet. 1989 Mar;81(4):308–310. doi: 10.1007/BF00283680. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Smeets H., Lambermon H. M., Coerwinkel-Driessen M., van Oost B. A., Wieringa B., Ropers H. H. A multipoint linkage map around the locus for myotonic dystrophy on chromosome 19. Genomics. 1989 Oct;5(3):589–595. doi: 10.1016/0888-7543(89)90027-x. [DOI] [PubMed] [Google Scholar]
- Bufton L., Bruns G. A., Magenis R. E., Tomar D., Shaw D., Brook D., Litt M. Four restriction fragment length polymorphisms revealed by probes from a single cosmid map to chromosome 19. Am J Hum Genet. 1986 Apr;38(4):447–460. [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987 May 15;262(14):6460–6463. [PubMed] [Google Scholar]
- Coerwinkel-Driessen M., Schepens J., van Zandvoort P., van Oost B., Mariman E., Wieringa B. NcoI RFLP at the creatine kinase-muscle type gene locus (CKMM, chromosome 19). Nucleic Acids Res. 1988 Sep 12;16(17):8743–8743. doi: 10.1093/nar/16.17.8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W., Harbitz I., Fries R., Stranzinger G., Hauge J. G. Porcine malignant hyperthermia carrier detection and chromosomal assignment using a linked probe. Anim Genet. 1988;19(3):203–212. doi: 10.1111/j.1365-2052.1988.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gronert G. A. Malignant hyperthermia. Anesthesiology. 1980 Nov;53(5):395–423. doi: 10.1097/00000542-198011000-00007. [DOI] [PubMed] [Google Scholar]
- Hojný J., Cepica S., Hradecký J. Gene order and recombination rates in the linkage group S-Phi-Hal-H-(Po2)-Pgd in pigs. Anim Blood Groups Biochem Genet. 1985;16(4):307–318. doi: 10.1111/j.1365-2052.1985.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Juneja R. K., Gahne B., Stratil A. Polymorphic plasma postalbumins of some domestic animals (pig PO2, horse Xk and dog Pa proteins) identified as homologous to human plasma alpha 1B-glycoprotein. Anim Genet. 1987;18(2):119–124. doi: 10.1111/j.1365-2052.1987.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Kim D. H., Sreter F. A., Ohnishi S. T., Ryan J. F., Roberts J., Allen P. D., Meszaros L. G., Antoniu B., Ikemoto N. Kinetic studies of Ca2+ release from sarcoplasmic reticulum of normal and malignant hyperthermia susceptible pig muscles. Biochim Biophys Acta. 1984 Sep 5;775(3):320–327. doi: 10.1016/0005-2736(84)90187-1. [DOI] [PubMed] [Google Scholar]
- Korneluk R. G., MacKenzie A. E., Nakamura Y., Dubé I., Jacob P., Hunter A. G. A reordering of human chromosome 19 long-arm DNA markers and identification of markers flanking the myotonic dystrophy locus. Genomics. 1989 Oct;5(3):596–604. doi: 10.1016/0888-7543(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Korneluk R. G., MacLeod H. L., McKeithan T. W., Brooks J. D., MacKenzie A. E. A chromosome 19 clone from a translocation breakpoint shows close linkage and linkage disequilibrium with myotonic dystrophy. Genomics. 1989 Feb;4(2):146–151. doi: 10.1016/0888-7543(89)90293-0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J., Heinzmann C., Sparkes R. S., Scott J., Knott T. J., Geller R., Sparkes M. C., Mohandas T. Regional mapping of human chromosome 19: organization of genes for plasma lipid transport (APOC1, -C2, and -E and LDLR) and the genes C3, PEPD, and GPI. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3929–3933. doi: 10.1073/pnas.83.11.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A. E., MacLeod H. L., Hunter A. G., Korneluk R. G. Linkage analysis of the apolipoprotein C2 gene and myotonic dystrophy on human chromosome 19 reveals linkage disequilibrium in a French-Canadian population. Am J Hum Genet. 1989 Jan;44(1):140–147. [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Champaneria S., Holland P. C., Powers V. E., Willard H. F. Fast-twitch and slow-twitch/cardiac Ca2+ ATPase genes map to human chromosomes 16 and 12. Somat Cell Mol Genet. 1987 Jul;13(4):341–346. doi: 10.1007/BF01534928. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990 Feb 8;343(6258):559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Mickelson J. R., Gallant E. M., Litterer L. A., Johnson K. M., Rempel W. E., Louis C. F. Abnormal sarcoplasmic reticulum ryanodine receptor in malignant hyperthermia. J Biol Chem. 1988 Jul 5;263(19):9310–9315. [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Hellkuhl B., Grzeschik K. H., Shapiro L. J. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6759–6763. doi: 10.1073/pnas.77.11.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. E. Abnormality in calcium release from skeletal sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. J Clin Invest. 1983 Sep;72(3):862–870. doi: 10.1172/JCI111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P. J. Porcine malignant hyperthermia susceptibility: hypersensitive calcium-release mechanism of skeletal muscle sarcoplasmic reticulum. Can J Vet Res. 1986 Jul;50(3):318–328. [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schepens J., Smeets H., Hulsebos T., Brunner H., Wieringa B. Isolation of a polymorphic DNA sequence pJSB11 (D19S16) from the human chromosome 19cen-q13.2 region linked to the myotonic dystrophy (DM) gene. Nucleic Acids Res. 1987 Apr 10;15(7):3192–3192. doi: 10.1093/nar/15.7.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonk D., Coerwinkel-Driessen M., van Dalen I., Oerlemans F., Smeets B., Schepens J., Hulsebos T., Cockburn D., Boyd Y., Davis M. Definition of subchromosomal intervals around the myotonic dystrophy gene region at 19q. Genomics. 1989 Apr;4(3):384–396. doi: 10.1016/0888-7543(89)90346-7. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Sidoli A., Shelley C. S., Lucero M. A., Shoulders C. C., Baralle F. E. Human apolipoproteins AI, AII, CII and CIII. cDNA sequences and mRNA abundance. Nucleic Acids Res. 1984 May 11;12(9):3917–3932. doi: 10.1093/nar/12.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Meredith A. L., Sarfarazi M., Harley H. G., Huson S. M., Brook J. D., Bufton L., Litt M., Mohandas T., Harper P. S. Regional localisations and linkage relationships of seven RFLPs and myotonic dystrophy on chromosome 19. Hum Genet. 1986 Nov;74(3):262–266. doi: 10.1007/BF00282545. [DOI] [PubMed] [Google Scholar]
- Smeets H., Markslag P., Bril J., Hulsebos T., Brunner H., Schonk D., Ropers H. H., Wieringa B. EcoRI RFLP at 19 cen-q13.2 identified by the anonymous DNA sequence pPM6.7 (D19S18). Nucleic Acids Res. 1987 Oct 12;15(19):8120–8120. doi: 10.1093/nar/15.19.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- Wallis S. C., Donald J. A., Forrest L. A., Williamson R., Humphries S. E. The isolation of a genomic clone containing the apolipoprotein CII gene and the detection of linkage disequilibrium between two common DNA polymorphisms around the gene. Hum Genet. 1984;68(4):286–289. doi: 10.1007/BF00292585. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]