Abstract
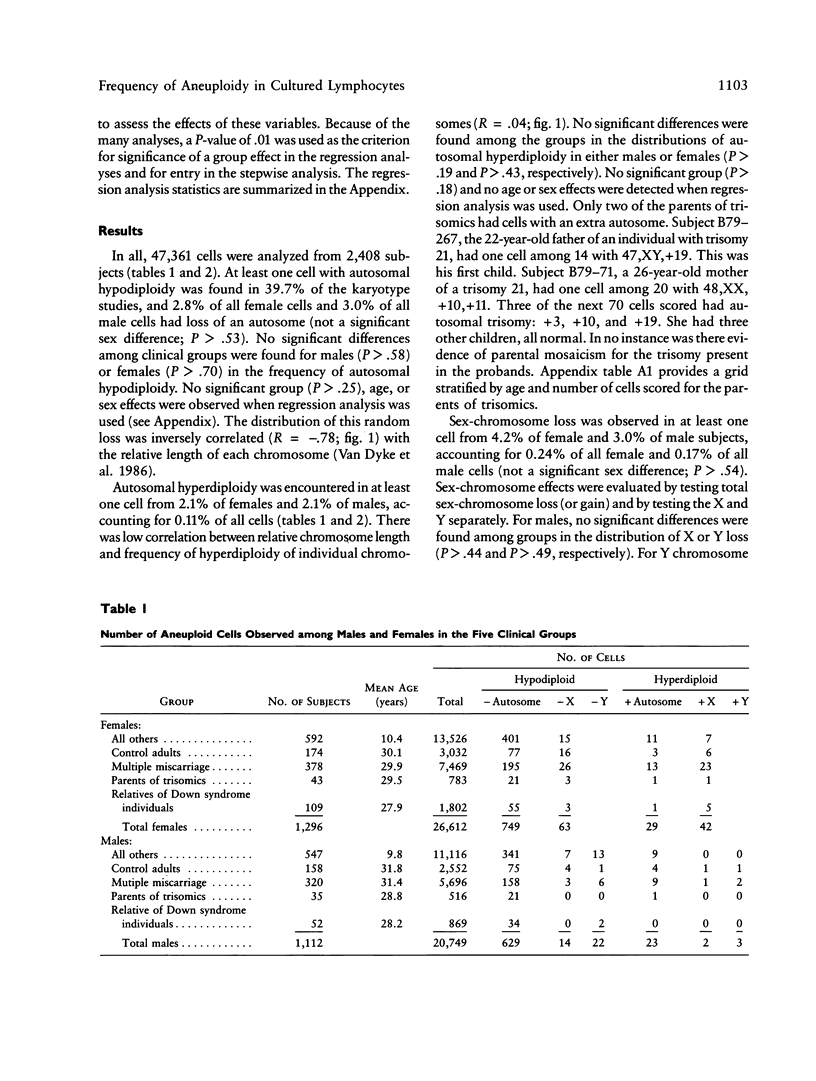
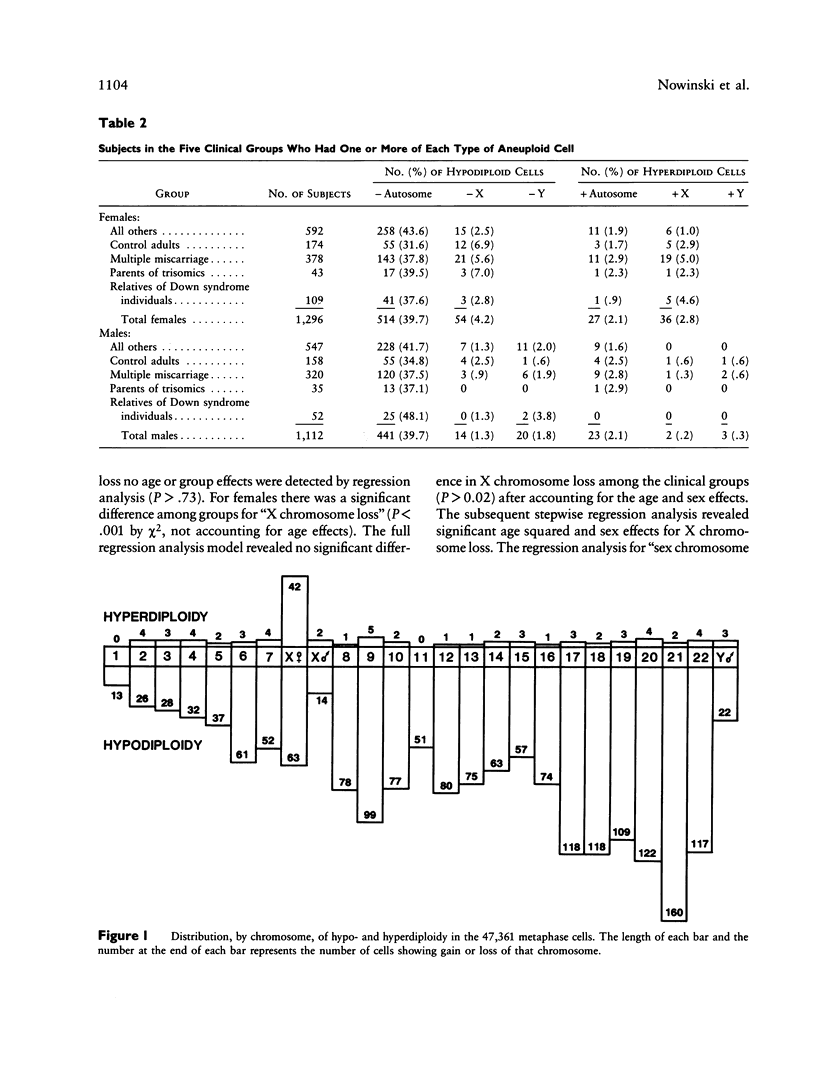
The clinical significance of low numbers of aneuploid cells in routine cytogenetic studies of cultured lymphocytes is not always clear. We compared the frequencies of chromosome loss and gain among five groups of subjects whose karyotypes were otherwise normal; these groups were (1) subjects studied because of multiple miscarriages, (2) parents of live borns with autosomal trisomy, (3) subjects studied because they had a relative with Down syndrome, (4) an age-matched control group of phenotypically normal adults studied for other reasons (e.g., parent of a dysmorphic child or member of a translocation family), and (5) other mostly younger and phenotypically abnormal subjects who could not be assigned to the first four groups (e.g., individuals with multiple congenital anomalies or mental retardation). No significant age, sex, or group effects were observed for autosomal loss (hypodiploidy) or gain (hyperdiploidy). Autosomal loss was inversely correlated with relative chromosome length, but autosomal gain was not. Sex-chromosome gain was significantly more frequent in females than in males, but sex-chromosome loss was not significantly different between the sexes. Significant age effects were observed for both gain and loss of sex chromosomes. When age and sex were accounted for, the frequencies of sex-chromosome loss and gain were not significantly different among the five clinical groups. In general, low numbers of aneuploid cells are not clinically important when observed in blood chromosome preparations of subjects studied because of multiple miscarriages or a family history of autosomal trisomy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo M. A., Mayer M., Jacobs P. A. Aging and aneuploidy: evidence for the preferential involvement of the inactive X chromosome. Cytogenet Cell Genet. 1985;39(4):275–278. doi: 10.1159/000132157. [DOI] [PubMed] [Google Scholar]
- Brook J. D., Gosden R. G., Chandley A. C. Maternal ageing and aneuploid embryos--evidence from the mouse that biological and not chronological age is the important influence. Hum Genet. 1984;66(1):41–45. doi: 10.1007/BF00275184. [DOI] [PubMed] [Google Scholar]
- Brown T., Fox D. P., Robertson F. W., Bullock I. Non-random chromosome loss in PHA-stimulated lymphocytes from normal individuals. Mutat Res. 1983 Dec;122(3-4):403–406. doi: 10.1016/0165-7992(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Campana M., Serra A., Neri G. Role of chromosome aberrations in recurrent abortion: a study of 269 balanced translocations. Am J Med Genet. 1986 Jun;24(2):341–356. doi: 10.1002/ajmg.1320240214. [DOI] [PubMed] [Google Scholar]
- Castle D., Bernstein R. Cytogenetic analysis of 688 couples experiencing multiple spontaneous abortions. Am J Med Genet. 1988 Mar;29(3):549–556. doi: 10.1002/ajmg.1320290312. [DOI] [PubMed] [Google Scholar]
- Diedrich U., Hansmann I., Janke D., Opitz O., Probeck H. D. Chromosome anomalies in 136 couples with a history of recurrent abortions. Hum Genet. 1983;65(1):48–52. doi: 10.1007/BF00285027. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Archer S. A., Morris C. M. Evidence for the repeated primary non-disjunction of chromosome 21 as a result of premature centromere division (PCD). Hum Genet. 1986 Jan;72(1):58–62. doi: 10.1007/BF00278818. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., McEwan C. M. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet. 1977 Dec 23;39(3):329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Pickering A. F., Mercer J. M., Miethke P. M. Premature centromere division: a mechanism of non-disjunction causing X chromosome aneuploidy in somatic cells of man. Ann Hum Genet. 1975 May;38(4):417–428. doi: 10.1111/j.1469-1809.1975.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Ford J. H., Russell J. A. Differences in the error mechanisms affecting sex and autosomal chromosomes in women of different ages within the reproductive age group. Am J Hum Genet. 1985 Sep;37(5):973–983. [PMC free article] [PubMed] [Google Scholar]
- Ford J. H. Spindle microtubular dysfunction in mothers of Down syndrome children. Hum Genet. 1984;68(4):295–298. doi: 10.1007/BF00292587. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Buckton K. E. Aneuploidy and ageing: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet. 1978;20(1-6):78–95. doi: 10.1159/000130842. [DOI] [PubMed] [Google Scholar]
- Hamerton J. L., Taylor A. I., Angell R., McGuire V. M. Chromosome investigations of a small isolated human population: chromosome abnormalities and distribution of chromosome counts according to age and sex among the population of Tristan da Cunha. Nature. 1965 Jun 19;206(990):1232–1234. doi: 10.1038/2061232a0. [DOI] [PubMed] [Google Scholar]
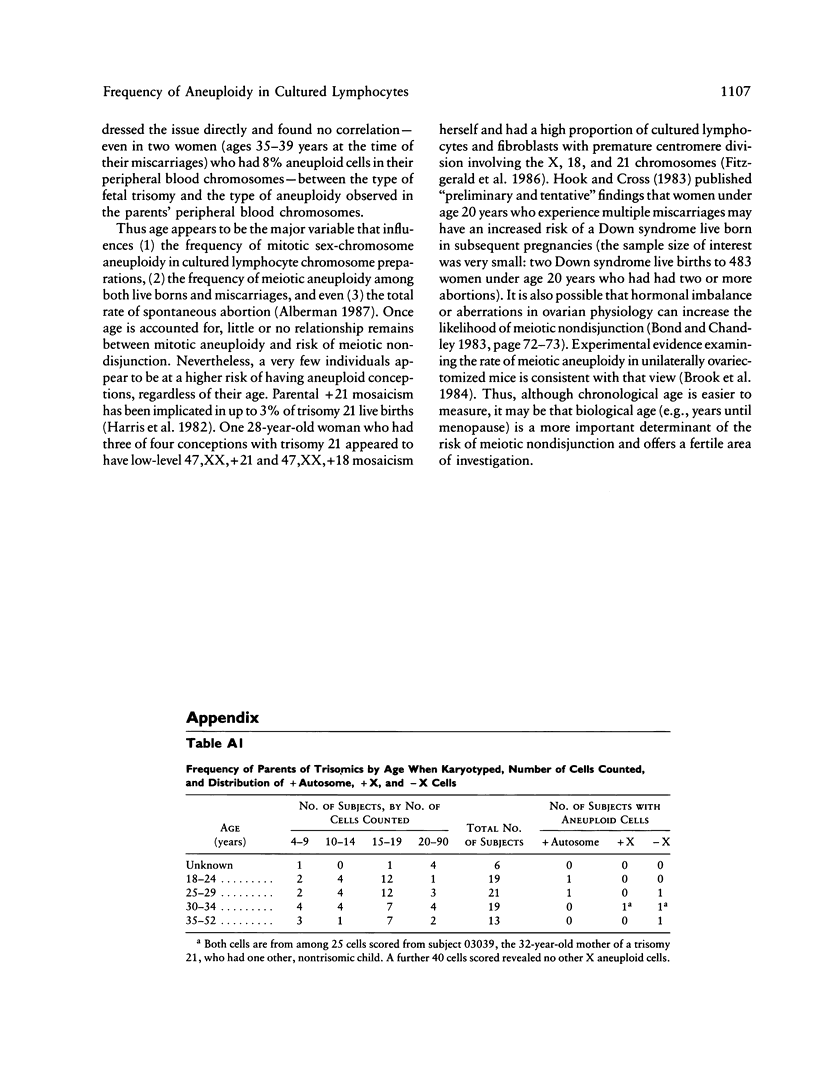
- Harris D. J., Begleiter M. L., Chamberlin J., Hankins L., Magenis R. E. Parental trisomy 21 mosaicism. Am J Hum Genet. 1982 Jan;34(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Jacobs P. A., Pettay D. Cytogenetic studies of couples with repeated spontaneous abortions of known karyotype. Genet Epidemiol. 1988;5(2):65–74. doi: 10.1002/gepi.1370050202. [DOI] [PubMed] [Google Scholar]
- Hecht F., Hecht B. K., Berger C. S. Aneuploidy in recurrent spontaneous aborters: the tendency to parental nondisjunction. Clin Genet. 1984 Jul;26(1):43–45. doi: 10.1111/j.1399-0004.1984.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Holzgreve W., Schonberg S. A., Douglas R. G., Golbus M. S. X-chromosome hyperploidy in couples with multiple spontaneous abortions. Obstet Gynecol. 1984 Feb;63(2):237–240. [PubMed] [Google Scholar]
- Hook E. B., Cross P. K. Spontaneous abortion and subsequent Down syndrome livebirth. Hum Genet. 1983;64(3):267–270. doi: 10.1007/BF00279407. [DOI] [PubMed] [Google Scholar]
- Horsman D. E., Dill F. J., McGillivray B. C., Kalousek D. K. X chromosome aneuploidy in lymphocyte cultures from women with recurrent spontaneous abortions. Am J Med Genet. 1987 Dec;28(4):981–987. doi: 10.1002/ajmg.1320280425. [DOI] [PubMed] [Google Scholar]
- JACOBS P. A., BRUNTON M., BROWN W. M. CYTOGENETIC STUDIES IN LEUCOCYTES ON THE GENERAL POPULATION: SUBJECTS OF AGES 65 YEARS AND MORE. Ann Hum Genet. 1964 Jun;27:353–365. doi: 10.1111/j.1469-1809.1963.tb01532.x. [DOI] [PubMed] [Google Scholar]
- JACOBS P. A., BRUNTON M., COURT BROWN W. M., DOLL R., GOLDSTEIN H. Change of human chromosome count distribution with age: evidence for a sex differences. Nature. 1963 Mar 16;197:1080–1081. doi: 10.1038/1971080a0. [DOI] [PubMed] [Google Scholar]
- JACOBS P. A., COURT BROWN W. M., DOLL R. Distribution of human chromosome counts in relation to age. Nature. 1961 Sep 16;191:1178–1180. doi: 10.1038/1911178a0. [DOI] [PubMed] [Google Scholar]
- Jarvik L. F., Kato T. Chromosome examinations in aged twins. Am J Hum Genet. 1970 Sep;22(5):562–573. [PMC free article] [PubMed] [Google Scholar]
- Jarvik L. F., Yen F. S., Fu T. K., Matsuyama S. S. Chromosomes in old age: a six year longitudinal study. Hum Genet. 1976 Jul 7;33(1):17–22. doi: 10.1007/BF00447282. [DOI] [PubMed] [Google Scholar]
- Jarvik L. F., Yen F. S., Goldstein F. Chromosomes and mental status. A study of women residing in institutions for the elderly. Arch Gen Psychiatry. 1974 Feb;30(2):186–190. doi: 10.1001/archpsyc.1974.01760080046007. [DOI] [PubMed] [Google Scholar]
- Juberg R. C., Knops J., Mowrey P. N. Increased frequency of lymphocytic mitotic non-disjunction in recurrent spontaneous aborters. J Med Genet. 1985 Feb;22(1):32–35. doi: 10.1136/jmg.22.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattevi M. S., Salzano F. M. Senescence and human chromosome changes. Humangenetik. 1975;27(1):1–8. doi: 10.1007/BF00283497. [DOI] [PubMed] [Google Scholar]
- Meyers C. M., Simpson J. L., Martin A. O., Elias S. Hypermodal cells, hypomodal cells, and repetitive abortions. Fertil Steril. 1986 Oct;46(4):615–618. [PubMed] [Google Scholar]
- Michels V. V., Medrano C., Venne V. L., Riccardi V. M. Chromosome translocations in couples with multiple spontaneous abortions. Am J Hum Genet. 1982 May;34(3):507–513. [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Martin J. M., Kahn J. A mathematical model predicting chromosome loss in cultured cells of young adults. J Theor Biol. 1978 Jul 20;73(2):237–245. doi: 10.1016/0022-5193(78)90188-1. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Chromosomes in senile dementia. Br J Psychiatry. 1968 Mar;114(508):303–309. doi: 10.1192/bjp.114.508.303. [DOI] [PubMed] [Google Scholar]
- Osztovics M. K., Tóth S. P., Wessely J. A. Cytogenetic investigations in 418 couples with recurrent fetal wastage. Ann Genet. 1982;25(4):232–236. [PubMed] [Google Scholar]
- Pantzar J. T., Allanson J. E., Kalousek D. K., Poland B. J. Cytogenetic findings in 318 couples with repeated spontaneous abortion: a review of experience in British Columbia. Am J Med Genet. 1984 Mar;17(3):615–620. doi: 10.1002/ajmg.1320170310. [DOI] [PubMed] [Google Scholar]
- Pierre R. V., Hoagland H. C. 45,X cell lines in adult men: loss of Y chromosome, a normal aging phenomenon? Mayo Clin Proc. 1971 Jan;46(1):52–55. [PubMed] [Google Scholar]
- Sachs E. S., Jahoda M. G., Van Hemel J. O., Hoogeboom A. J., Sandkuyl L. A. Chromosome studies of 500 couples with two or more abortions. Obstet Gynecol. 1985 Mar;65(3):375–378. [PubMed] [Google Scholar]
- Sandberg A. A., Cohen M. M., Rimm A. A., Levin M. L. Aneuploidy and age in a population survey. Am J Hum Genet. 1967 Sep;19(5):633–643. [PMC free article] [PubMed] [Google Scholar]
- Smith A., Elliott G. Aneuploidy in culture. J Ment Defic Res. 1980 Sep;24(3):159–165. doi: 10.1111/j.1365-2788.1980.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Staessen C., Maes A. M., Kirsch-Volders M., Susanne C. Is there a predisposition for meiotic nondisjunction that may be detected by mitotic hyperploidy? Clin Genet. 1983 Sep;24(3):184–190. [PubMed] [Google Scholar]
- Stallard R., Haney N. R., Frank P. A., Styron P., Juberg R. C. Leukocyte chromosomes from parents of cytogenetically abnormal offspring: preliminary observations. Cytogenet Cell Genet. 1981;30(1):50–53. doi: 10.1159/000131588. [DOI] [PubMed] [Google Scholar]
- Van Dyke D. L., Worsham M. J., Fisher L. J., Weiss L. The centromere index and relative length of human high-resolution G-banded chromosomes. Hum Genet. 1986 Jun;73(2):130–132. doi: 10.1007/BF00291602. [DOI] [PubMed] [Google Scholar]
- Wenger S. L., Golden W. L., Dennis S. P., Steele M. W. Are the occasional aneuploid cells in peripheral blood cultures significant? Am J Med Genet. 1984 Dec;19(4):715–719. doi: 10.1002/ajmg.1320190411. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A. How do human isochromosomes arise? Cancer Genet Cytogenet. 1982 Feb;5(2):173–179. doi: 10.1016/0165-4608(82)90007-3. [DOI] [PubMed] [Google Scholar]


