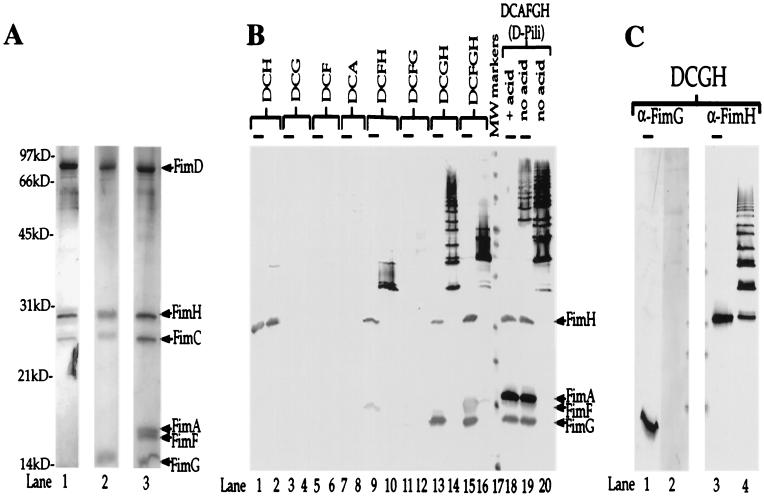
Figure 2.
Multimerization events demonstrate molecular specificity of subunit-subunit interactions. (A) Silver-stained gel (Bio-Rad Silver Stain Plus). Outer membrane preparations were subjected to nickel chromatography (to purify the His-tagged FimD) after coexpression with various combinations of pilus subunits. Samples were boiled in SDS sample buffer and subjected to standard SDS/PAGE. Protein samples purified from E. coli expressing only FimDCH were run in lane 1; those expressing only FimDCGH were run in lane 2; and those expressing FimDCAFGH were run in lane 3. Arrows and labels indicate the expected molecular masses of each of the copurifying proteins: FimD, 92 kDa; FimH, 29 kDa; FimC, 26 kDa; FimA, 18 kDa; FimF, 17 kDa; and FimG, 15 kDa. kD, kilodalton. (B) Anti-type 1 pilus Western blot of samples (purified as those shown in A) either boiled (lanes with overlying bars: odd numbered lanes 1–15 and lanes 18 and 19) or kept at room temperature (even numbered lanes 2–20 with the exception of lane 18) in SDS sample buffer before standard SDS/PAGE. Based on the behavior of whole pili in this assay (lanes 18–20) and previous studies (10), higher-molecular-mass ladders represent higher-order multimers. (C) Western blot of the same samples analyzed in B, lanes 13 and 14, except the blot was developed with anti-FimG antiserum (prepared against an N-terminal 21-amino acid peptide; lanes 1 and 2) and anti-FimH antiserum (lanes 3 and 4). Lanes 2 and 4 contain unboiled samples. Lanes 1 and 3 contain boiled samples (lanes with overlying bars). We hypothesize that the N terminus of FimG is hidden in the FimH-FimG multimer, because it is likely involved in head-to-tail donor strand exchange interactions (22–24, 31).