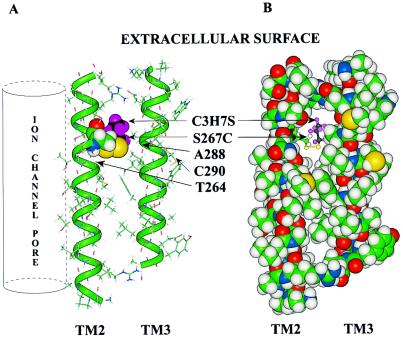
Figure 7.
Molecular model of TM2 and TM3 of the GlyR α1 subunit. TM2 is oriented so that the aqueous pore of the ion channel would be on the far left (vertical cylinder). This orientation places residue S267C on the interior of the subunit. The TM3 helix was aligned anti-parallel to TM2 so that S267 and A288 would be on the same horizontal plane. (A) The peptides are rendered as a stick structure, the backbones of the α helices are overlaid with a green tube, and both S267C and propanethiol are rendered with a space-filling (van der Waals) surface. The colors of atoms are: carbon, green; hydrogen, gray; oxygen, red; nitrogen, blue; and the propyl group of propanethiol is highlighted with black carbons and violet hydrogens. Those cysteine residues that did not form covalent bonds with PMTS or that formed them without any effect on potentiation of the effect of glycine are indicated (T264C, A288C, and C290). (B) The same molecular model of TM2 and TM3 is shown except that all residues in the two helices are rendered with a space-filling surface and the propanethiol is rendered as a ball-and-stick molecule. It can be seen that the propyl group occupies the cavity and may prevent the additional occupation of the cavity by octanol, isoflurane, or enflurane.