Figure 1.
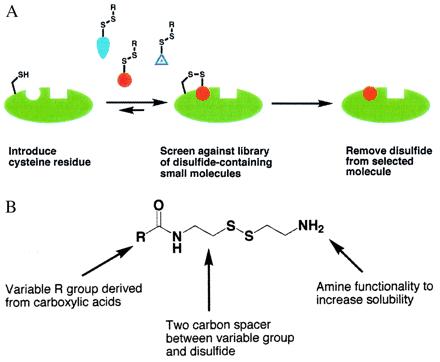
(A) Schematic illustration of the tethering approach: a cysteine-containing protein is equilibrated with a disulfide-containing library in the presence of a reducing agent such as 2-mercaptoethanol. Most of the library members will have little or no inherent affinity for the protein, and thus by mass action the equilibrium will lie toward the unmodified protein. However, if a library member does show inherent affinity for the protein, the equilibrium will shift toward the modified protein. (B) Schematic illustration of a generic disulfide library derived from carboxylic acids. Other functional groups have also been converted to disulfide libraries, as described in Materials and Methods. In the present case, 1,200 compounds were screened against TS in pools of 8 to 15 compounds.
