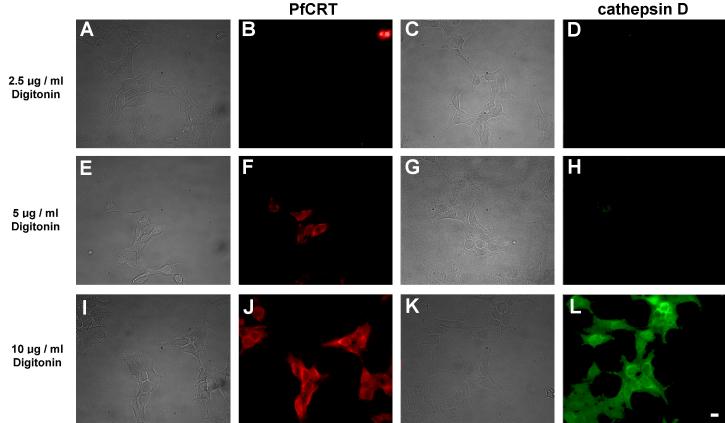
Fig. 3.
Demonstration of selective plasma membrane permeabilization by digitonin. HEK293 cells transfected with Dd2-bad were fixed and permeabilized with digitonin at the concentration indicated on the left of each row. Bright field images are shown on the left hand side of each corresponding epifluorescence image. (B, F, J) Red fluorescence from Cy3-streptavidin. (D, H, L) Green fluorescence from cathepsin D labeled with anti-cathepsin primary antibodies and Alexa Fluor 488 secondary antibodies. For cells treated with 5 μg/ml digitonin, note the red fluorescence in panel F indicating detection of the biotinylated PfCRT C-terminal fusion biotin-acceptor domain, and the corresponding lack of green fluorescence in panel H at the same digitonin concentration indicating the inaccessibility of intralysosomal cathepsin D to the anti-cathepsin antibodies. This indicates that the plasma membrane has been permeabilized but the lysosomal membrane has not been permeabilized. The ability of Cy3-streptavidin to bind to the C-terminal fusion biotin-acceptor domain under these conditions indicates that PfCRT is embedded in the limiting membrane of the lysosomes with its C-terminus in the cytoplasm. At 10 μg/ml digitonin the lysosomal membrane was permeabilized and the anti-cathepsin antibodies show extensive labeling (L). Scale bar = 10 μm.