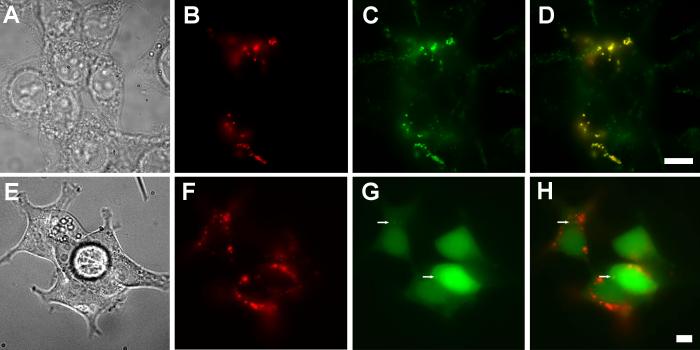
Fig. 7.
Attempted perturbation of PfCRT sorting. Truncation of the N- and C-terminal domains does not alter PfCRT trafficking to lysosomes nor does coexpression of PfCRT with LC3-GFP. (A-D) HB3-NTD-CTD-myc PfCRT that lacks the N- and C-terminal cytoplasmic domains still traffics to the LAMP-2 positive lysosomal compartment. (A) Bright field, (B; red) HB3-NTD-CTD-myc, detected via indirect immunofluorescence with an anti-myc-tag antibody, biotinylated-anti-rabbit secondary and Cy3-streptavidin. (C; green) LAMP-2, detected via H4B4 antibodies and anti-mouse Alexa Fluor 488 secondary antibodies. (D) Overlay of panels B and C showing colocalization of LAMP-2 and HB3-NTD-CTD-myc. (E-H) Illustrates that overexpression of LC3, which causes an increase in autophagic vesicle formation, does not alter PfCRT localization. (E) Bright field, (F, red) detected via indirect immunofluorescence with anti-myc-tag antibodies, biotinylated-anti-rabbit secondary and Cy3-streptavidin; (G; green) Green fluorescence from LC3-GFP. (H) Overlay of panels F and G. Arrows indicate punctae of LC3-GFP. Scale bar = 10 μm.