Figure 1.
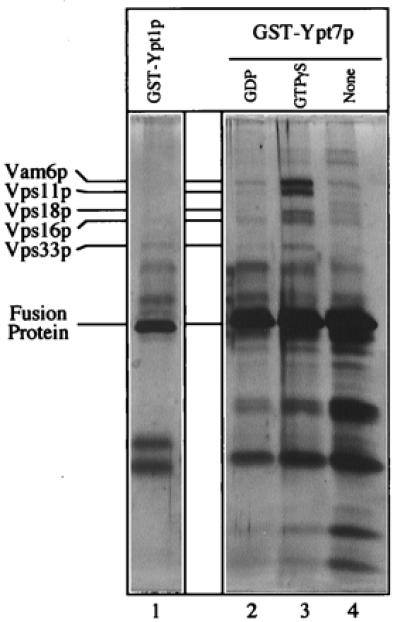
The class C Vps proteins associate with glutathione-immobilized GST-Ypt7p:GTP[γS]. Frozen BJ3505 vacuoles (300 μg) were diluted to 1.5 ml with PS buffer (see Materials and Methods) and sedimented (13,000 × g, 10 min, 4°C). Vacuole pellets were suspended in 500 μl of IP solubilization buffer [20 mM Hepes, pH 7.4/150 mM NaCl/10% glycerol/1% Triton X-100/1× protease inhibitor mixture (PIC; ref. 30)] and incubated on ice for 10 min. After sedimentation (13,000 × g, 10 min, 4°C), the solubilized vacuolar supernatant was transferred to fresh tubes containing GST-Ypt1p (lane 1) or GST-Ypt7p (lanes 2–4) that had been prebound to 40 μl (packed volume) of glutathione-Sepharose beads (Amersham Pharmacia) and preloaded with nucleotides (see below). [Fusion proteins underwent nucleotide exchange before incubation with the solubilized vacuolar supernatants. Each fusion protein (120 μg) was mixed by nutation with 40 μl of glutathione-Sepharose beads in 50 mM Tris·Cl, pH 8.0, for 20 min at room temperature. Bound material was sedimented (10,000 × g, 1 min, 4°C), and the supernatant was decanted. Beads were incubated (20 min, room temperature) in 500 μl of PS elution buffer (PS buffer with 2 M NaCl, 20 mM EDTA, 5 mM GDP, 1 mM DTT, and 1× PIC) and then sedimented and washed four times by resuspension in 1 ml of PS buffer and sedimentation (10,000 × g, 1 min, 4°C). Beads with fusion proteins were incubated (20 min, room temperature) with either 4 mM GTP[γS] (lanes 1 and 3), 4 mM GDP (lane 2), or no nucleotide (lane 4) in 500 μl of PS loading buffer (PS buffer containing 150 mM KCl, 4.5 mM MgCl2, 0.5 mM MnCl2, 1 mM DTT, and 1× PIC) and collected (10,000 × g, 1 min, 4°C).] Detergent extracts of vacuoles were mixed with beads by nutation for 2 h at 4°C. Unbound material was decanted from the beads after centrifugation (10,000 × g, 1 min, 4°C). Beads were resuspended twice in 1 ml of IP solubilization buffer and sedimented as above, and then bound proteins were eluted with 500 μl of PS elution buffer. Elution was by nutation for 15 min at room temperature followed by centrifugation. The eluate was precipitated by 12.5% trichloroacetic acid for 15 min on ice followed by centrifugation (13,000 × g, 15 min, 4°C). The pellet was resuspended in 1 ml of 80% acetone and sedimented as above. Protein pellets were dried and resuspended in 50 μl SDS/PAGE loading buffer, and polypeptides were separated on 10% acrylamide gels (35). Visualization was by silver staining (36); mass spectroscopy analysis (at the Howard Hughes Medical Institute/Keck Lab, Yale University) was performed on polypeptides extracted from a similar preparative gel stained with Coomassie brilliant blue.
