Figure 3.
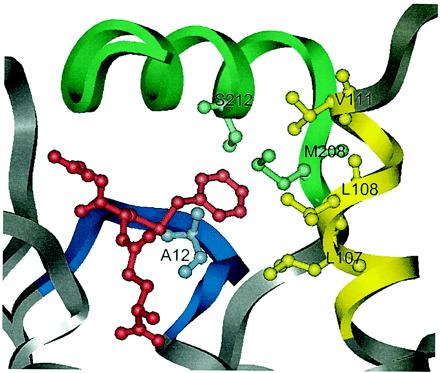
Close view of the active site of GST A1–1 (2). The ligand, S-benzylglutathione, which defines the position of the active site, is colored red. The following mutations have been introduced in mutant GIMFhelix: Ala-12–Gly in the β1-α1 loop (blue) and Leu-107–Ile, Leu-108–Met, and Val-111–Phe in the C-terminal part of α4-helix (yellow). The C terminus (green) of GST A1–1 has been replaced with the corresponding residues of GST A4–4. This substitution altered 10 amino acid residues and included the mutations Ser-212–Tyr and Met-208–Pro, believed to be important for the change of the mechanism to a Michael addition.
