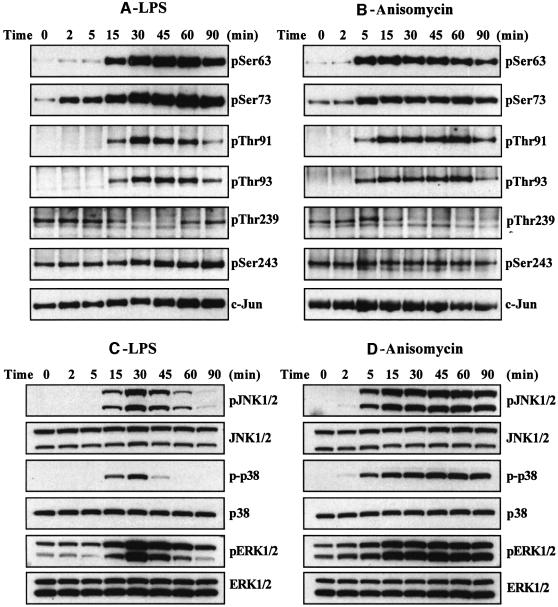
Fig. 2. Effect of LPS and anisomycin on the phosphorylation of six sites on c-Jun in RAW macrophages. Macrophages were stimulated with LPS (100 ng/ml) or anisomycin (10 µg/ml) for the times indicated and lysed. c-Jun was immunoprecipitated from the lysates denatured in SDS, subjected to SDS–PAGE and transferred to nitrocellulose. The membranes were immunoblotted with antibodies that recognize each of the six phosphorylation sites on c-Jun, as well as with an antibody that recognizes the phosphorylated and unphosphorylated forms of c-Jun equally well (A and B). Further aliquots of the lysates were immunoblotted with antibodies that recognize the active phosphorylated forms of several MAP kinases, namely JNK isoforms, p38α and ERK1 and ERK2, as well as with antibodies that recognize the phosphorylated and dephosphorylated forms of these kinases equally well (C and D). The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.