Abstract
The evolution of the microbial spoilage population for air- and vacuum-packaged meat (beef and pork) stored at 4°C was investigated over 11 days. We monitored the viable counts (mesophilic total aerobic bacteria, Pseudomonas spp., Enterobacteriaceae, lactic acid bacteria, and Enterococcus spp.) by the microbiological standard technique and by measuring the emission of volatile organic compounds (VOCs) with the recently developed proton transfer reaction mass spectrometry system. Storage time, packaging type, and meat type had statistically significant (P < 0.05) effects on the development of the bacterial numbers. The concentrations of many of the measured VOCs, e.g., sulfur compounds, largely increased over the storage time. We also observed a large difference in the emissions between vacuum- and air-packaged meat. We found statistically significant strong correlations (up to 99%) between some of the VOCs and the bacterial contamination. The concentrations of these VOCs increased linearly with the bacterial numbers. This study is a first step toward replacing the time-consuming plate counting by fast headspace air measurements, where the bacterial spoilage can be determined within minutes instead of days.
Meat is one of the most perishable foods, and its composition is ideal for the growth of a wide range of spoilage bacteria. Public concern has risen due to numerous food scandals such as those surrounding bovine spongiform encephalopathy and foot-and-mouth disease epidemics (8, 9, 19), and food-borne diseases remain a substantial burden (21). We can meet these challenges with an improved and global food safety control system. One possible improvement would be a rapid and accurate detection system for microbial spoilage. This technique should ideally also be nondestructive and give results in real time for application in highly automated food-processing environments. Current methods are time-consuming, labor intensive, and, therefore, give retrospective information (8). The common method used for determining the status of meat, with respect to spoilage, is analysis of the counts of total viable bacteria and/or specific spoilage bacteria. An obvious drawback with this method is the incubation period of 1 to 3 days that is required for colony formation. For enrichment cultures several days are needed. Molecular methods have been described as useful approaches to type bacteria and monitor community development in meat (27); quantification of microbial numbers, however, is not yet feasible. Therefore we explore here a novel and very fast method to determine the status of a meat sample within a few minutes to make real-time meat controls possible. Volatile organic compounds (VOCs) produced by meat bacteria have been analyzed by gas chromatography-mass spectrometry (MS) (5, 7) and have been detected by an electronic nose and a sensory panel (3), suggesting the helpfulness of VOC measurements in order to analyze spoilage. The objective of the present work was to evaluate the ability of the proton transfer reaction (PTR)-MS technique to detect VOCs which may be associated with the microbiological spoilage of cold-stored meat.
MATERIALS AND METHODS
Preparation of meat.
Fresh meat samples from the beef and pork shoulder areas (each approximately 2 and 7 kg, respectively) were cut into small pieces by a commercial meat-processing facility. The pieces all had about the same shape (weight, ca. 10 g; length, ca. 10 cm; width, ca. 1 cm; depth, ca. 1 cm). To get similar loadings of bacteria, all cuts of each meat type were thoroughly rubbed together for 2 min to distribute the bacteria from the different cuts over all the surfaces.
Packaging and storage.
Two packaging procedures were performed. Half of the meat pieces of beef and pork were air packaged individually in oxygen-permeable polyethylene film, and half of them were vacuum packaged individually in vacuum bagging film (polyamide-polyethylene [Packartis], with O2 and CO2 transmission rates of 10 and 35 cm3 m−2 24 h−1 105 Pa−1, respectively, at 23°C) by evacuating the package (97 to 99% vacuum) and sealing. The packaged meat pieces were immediately stored at 4°C. Immediately after packaging (time zero) and at daily intervals, up to 11 days, three meat pieces were withdrawn for each type of meat and each type of packaging for analysis.
Emission of VOCs.
For analyzing the VOCs we used a standard PTR-MS system which has been developed in our institute and which can be supplied by Ionicon Analytik GmbH, Innsbruck, Austria. The system allows an on-line measurement of trace components with concentrations as low as a few parts per trillion by volume. The method is based on ionizing reactions of H3O+ ions with the VOCs to be detected by nondissociative proton transfer. Most of the common VOCs react with H3O+, whereas the other major components present in clean air do not react. The generation of the primary H3O+ and the chemical ionization of the VOCs are individually controlled and spatially and temporally separated processes. One important consequence is that absolute headspace concentrations can be calculated without calibration or use of standards (26). Another big advantage of PTR-MS is that the samples containing the volatile compounds do not need any preparation (presampling, preconcentration, or sample dehydration) before being admitted to the PTR-MS. Thus some problems inherent to sampling in alternative methods used so far (e.g., gas chromatography) are avoided, the food itself is not disturbed, and the measured mass-spectral profiles closely reflect genuine headspace distributions (26). The PTR-MS system and measuring procedure has been described in detail previously (11, 15).
For measuring the VOCs meat pieces were unpackaged one by one under sterile conditions and placed in a glass vial with a volume of 35 ml that was then incubated at 25°C for 30 min to reach thermal equilibrium with the 25°C environment. Their headspace air was then drawn at 12 ml min−1 through a heated capillary into the PTR-MS system for on-line analysis. Synthetic air (Messer Austria; 20.5% O2 in N2) was used to generate the flow through the vial. The mass-spectrometric data were collected over a range of masses (m) with m/z values of 20 to 146 amu, where z is the charge of the measured ions (in our case z = 1). Instrument background concentrations of the VOCs were detected before the meat measurements and subtracted from the obtained emissions. Immediately after this analysis the meat pieces were transferred under sterile conditions into sterile 400-ml plastic bags (BagFilter P; Interscience) and frozen at −20°C for further analysis.
Identification of VOCs.
The quantity measured by PTR-MS is usually the intensity of a protonated compound, and for this compound information about its mass is obtained. Although there are a number of components having the same nominal mass, the number of possible compounds with the same mass is often drastically limited due to the origin of the air to be analyzed. Furthermore, there exist several methods to distinguish between isobars using PTR-MS (11, 15).
To assign the VOCs, information in addition to the observed mass was used. A vast literature on spoilage compounds in meat exists (1, 2, 4, 5, 7, 13, 20). This allows a first assignment of the prominent ion peaks. In addition, PTR-MS spectra of many pure VOCs were measured in order to assess their fragmentation patterns. Furthermore, the isotopic ratios for the assigned VOCs were checked. However, some superpositions occurred in the mass spectra as there were many peaks, and therefore we want to stress that for a definitive identification of the mass peaks in future follow-up studies a two-dimensional analysis such as gas chromatography-PTR-MS coupling is needed.
Microbiological analysis (enumeration of bacteria).
Meat pieces were thawed in the 400-ml plastic bags at room temperature (20°C). After 90 ml of a sterile solution consisting of 0.85% NaCl and 0.1% peptone (Oxoid; catalog no. LP0034) was added, the meat was homogenized in a stomacher (BagMixer W; Interscience) for 2 min at room temperature. Decimal dilutions in 0.85% NaCl-0.1% peptone were prepared, and 1- or 0.1-ml samples of appropriate dilutions were poured (plate count agar [PC], violet-red bile dextrose agar [VRBD], and Lactobacillus agar [MRS]) or spread (Slanetz and Bartley agar [SB] and Pseudomonas selective agar [GSP]) on the following media to determine microbial counts. Total viable aerobic counts were enumerated on PC (Merck; catalog no. 105463) incubated at 30°C for 48 h. The number of Pseudomonas spp. was determined on GSP as described by Kielwein (Merck; catalog no. 110230) supplemented with 100,000 IE of penicillin G (Calbiochem; catalog no. 5161) and natamycin (pimaricin; 0.01 g/liter; Merck; catalog no. 7360) and incubated at 30°C for 72 h; positive oxidase reactions were confirmed by using oxidase test strips (Bactident; Merck; catalog no. 113300). Lactic acid bacteria were enumerated on MRS as described by De Man et al. (Merck; catalog no. 110660) by incubation at 30°C for 72 h under microaerophilic conditions. The number of Enterobacteriaceae was determined on VRBD as described by Mossel (Merck; catalog no. 110275) by incubation at 37°C for 24 h. Enterococcus sp. counts were determined on SB (Oxoid; catalog no. CM0377) incubated at 37°C for 48 h; positive latex agglutination reactions were confirmed by using streptococcal latex grouping reagent D (Oxoid; catalog no. DR0589).
Statistical data analysis.
Normal distribution of the data was tested by the Kolmogorov-Smirnov test. Whether the meat type, the packaging type, or the storage time had a significant influence on bacterial numbers (normal distribution) was determined by analysis of variance (P < 0.05) and by multiple-range analysis (least significant difference). Correlations between bacterial numbers and the emitted VOCs during storage time were analyzed by the Pearson product moment correlation.
RESULTS
Microbiological analysis.
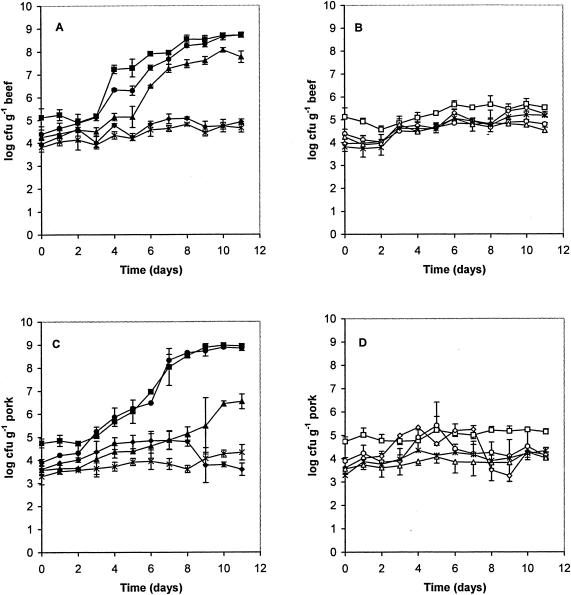
The bacterial counts, presented by plotting the log10 values of CFU in air- and vacuum-packaged beef and pork versus storage time at 4°C, are shown in Fig. 1. The initial bacterial numbers and the effect of packaging on the final numbers after 11 days of cold storage are presented in Table 1. Storage time, packaging type, and meat type had a statistically significant (P < 0.05) effect on the development of the microbial groups determined in this study. Large microbiological changes due to different biomass developments in the individual meat pieces (for example numbers of Enterobacteriaceae and lactic acid bacteria in air-packaged pork after 9 days; Fig. 1) resulted in higher standard deviations of bacterial counts.
FIG. 1.
Counts of total aerobic viable bacteria (▪, □), Pseudomonas spp. (•, ○), Enterobacteriaceae (▴, ▵), lactic acid bacteria (⧫, ◊), and Enterococcus spp. (×) in air-packaged (A and C; solid symbols) and vacuum-packaged (B and D; open symbols) beef (A and B) and pork (C and D) stored at 4°C. Each count is the mean of those for three individually packaged meat samples; error bars, standard deviations (n − 1).
TABLE 1.
Effect of air and vacuum packaging of beef and pork on bacterial counts after 11 days of storage at 4°C
| Bacterial group | Countsa (log CFU g−1) for:
|
|||||
|---|---|---|---|---|---|---|
| Beef
|
Pork
|
|||||
| Initial | After 11 days at 4°C, packaged in:
|
Initial | After 11 days at 4°C packaged in:
|
|||
| Air | Vacuum | Air | Vacuum | |||
| Aerobic | 5.13 ± 0.40 | 8.70 ± 0.10* | 5.53 ± 0.10 | 4.74 ± 0.20 | 8.94 ± 0.04* | 5.14 ± 0.12* |
| Pseudomonas spp. | 4.38 ± 0.20 | 8.72 ± 0.11* | 4.79 ± 0.07* | 3.91 ± 0.14 | 8.85 ± 0.11* | 4.10 ± 0.17 |
| Enterobacteriaceae | 4.25 ± 0.13 | 7.75 ± 0.25* | 4.52 ± 0.13 | 3.56 ± 0.17 | 6.53 ± 0.32* | 4.02 ± 0.07* |
| Lactic acid bacteria | 3.95 ± 0.05 | 4.91 ± 0.12* | 5.25 ± 0.11* | 3.60 ± 0.27 | 3.58 ± 0.27 | 4.15 ± 0.16* |
| Enterococcus spp. | 3.81 ± 0.20 | 4.65 ± 0.19* | 5.18 ± 0.06* | 3.29 ± 0.35 | 4.32 ± 0.34* | 4.33 ± 0.14* |
Values are means ± standard deviations (n − 1) for three individually package meat samples. ∗, significantly (P < 0.05) increased counts after 11 days compared to the initial counts.
(i) Effect of meat type.
The initial numbers of the total aerobic viable bacteria, lactic acid bacteria, and Enterococcus spp. in beef and pork were not significantly different. Beef had higher initial numbers of Pseudomonas spp. and Enterobacteriaceae than pork (Table 1). During 11 days of cold storage under air-packaged conditions, total viable counts as well as numbers of Pseudomonas spp. in beef and pork were not significantly different, whereas numbers of lactic acid bacteria, Enterobacteriaceae, and Enterococcus spp. were significantly higher in beef than in pork. If meat was vacuum packaged, bacterial numbers of all microbial groups determined in this study were significantly higher in beef than in pork.
(ii) Effect of packaging.
The type of packaging influenced the development of microbial numbers during 11 days at 4°C to similar extents in beef and pork. In both meat types the counts of total aerobic bacteria, Pseudomonas spp., and Enterobacteriaceae were considerably higher with air packaging than with vacuum packaging, while final counts of gram-positive bacteria, Enterococcus spp., and lactic acid bacteria were significantly higher when vacuum packaging was applied (Table 1).
In air-packaged meat (Fig. 1A and C), a significant increase of all bacterial groups during cold storage was found both in beef and pork. Total viable counts increased significantly after 2 to 3 days of storage at 4°C, and maximum numbers were detected after 10 to 11 days. Pseudomonas spp. were the dominant group in both types of meat, especially in pork, where their growth pattern was related to total viable counts after a delay of 2 days. The numbers of lactic acid bacteria, Enterobacteriaceae, and Enterococcus spp. were significantly higher in beef than in pork. While the numbers of Enterobacteriaceae increased with time (after a delay of 2 to 3 days) by a factor of about 103 in beef and pork, reaching a maximum after 10 to 11 days, the numbers of Enterococcus spp. increased only by a factor of about 10.
In vacuum-packaged meat (Fig. 1B and D), bacterial counts did not increase above 5 × 105 CFU g of meat−1 and were generally significantly higher in beef than in pork. When all counts obtained during 11 days were considered, there was a tendency toward significantly increased bacterial numbers. After 11 days at 4°C, total aerobic counts as well as numbers of Pseudomonas spp. and Enterobacteriaceae increased in both meat types by a factor of about 2 compared to initial values. Lactic acid bacteria and Enterococcus sp. populations multiplied by a factor of about 20 in beef but only three- to ninefold in pork (Table 1).
VOC analysis.
The aim of this work was to compare the measured VOC concentrations with the bacterial contamination. Therefore we first characterized the obtained mass spectra and tried to identify some compounds which typically appear during spoilage processes.
(i) Mass spectra.
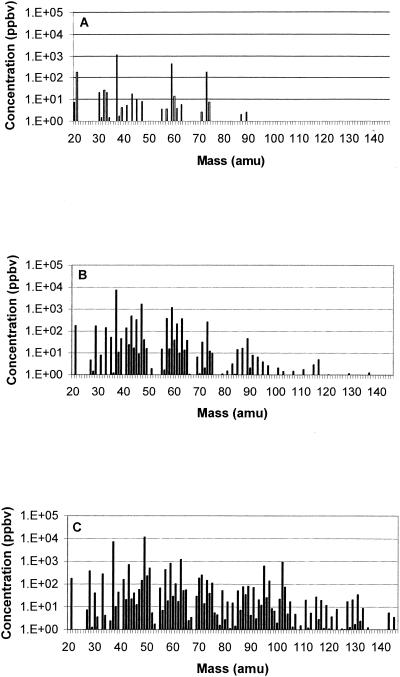
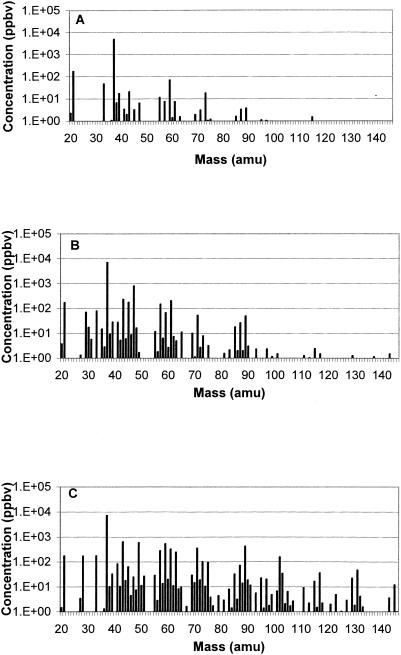
VOC concentrations averaged over the three replicates versus mass are shown for some of the meat samples in Fig. 2 and 3. Figure 2 compares the spectra of air- and vacuum-packaged beef samples at the beginning (day 0, Fig. 2A) and at the end (day 11, Fig. 2B and C) of the experiment. The concentrations of numerous components increased during storage time in vacuum-packaged (Fig. 2B) as well as in air-packaged (Fig. 2C) beef, but the increases of the emissions were much stronger for air-packaged samples. The mass spectra for pork (Fig. 3) showed the same trend as that for beef but there were less components and lower concentrations than for beef, especially for the air-packaged samples.
FIG. 2.
Mean VOC concentrations in beef at day 0 (A) and in vacuum-packaged beef (B) and air-packaged beef (C) after 11 days of storage at 4°C.
FIG. 3.
Mean VOC concentrations in pork at day 0 (A) and in vacuum-packaged pork (B) and air-packaged pork (C) after 11 days of storage at 4°C.
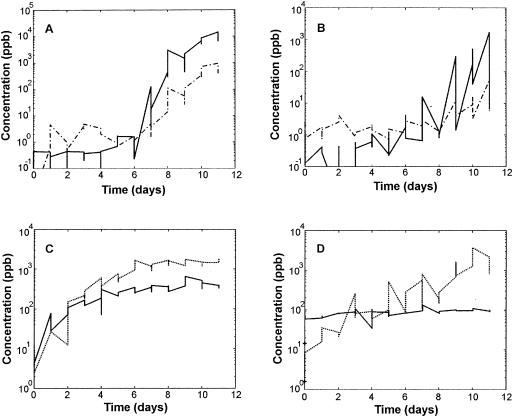
Figure 4 shows the concentrations of some typical spoiling compounds over the whole storage time. VOCs typically emitted by air-packaged meat were obtained at masses of 49, 63, and 95 amu (Fig. 4A and B), while VOCs with masses of 47 and 33 amu were found in vacuum-packaged meat (Fig. 4C and D).
FIG. 4.
Concentrations of typical VOCs emitted from air-packaged (A and B) and vacuum-packaged (C and D) beef (A and C) and pork (B and D) during 11 days of storage at 4°C (see Table 2 for assignment). Values of m+1 are as follows: 49 (A and B, solid line), 91 (A and B, dashed-dotted line), 47 (C and D, dashed line), and 33 amu (C and D, solid line).
(ii) Assignment of some VOCs.
Many peaks in the mass spectra could be tentatively identified with typical and well-known spoilage compounds (1, 2, 4, 5, 7, 13, 20). A few of those components which are relevant for this work (high correlation with bacterial numbers) are listed in Table 2, which also gives the protonated mass (m+1), the chemical formula, and a reference where this spoilage compound was identified. Note that due to ionizing the compounds via proton transfer in the PTR-MS we detect these compounds at the mass m+1. The assignment was based on measured fragmentation patterns, isotopic ratios, and published VOC compositions of spoiling meat.
TABLE 2.
List of relevant VOCs measured in the headspace of meat during 11 days of storage at 4°Ca
| m+1 (amu) | Compound(s) | Formula | Reference(s) |
|---|---|---|---|
| 33 | Methanol | CH4O | 2 |
| 47 | Ethanol | C2H6O | 1 |
| 49 | Methanethiol | CH4S | 5 |
| 57 | 1-Butanol (fragment) | C4H8 | 7 |
| 57 | 2-Methylpropan-2-ol (fragment) | C4H8 | 13, 20 |
| 63 | Dimethylsulfide | C2H6S | 1, 2, 4, 5 |
| 75 | Methylacetate | C3H6O2 | 20 |
| 91 | Diethylsulfide | C4H10S | 20 |
| 91 | Thioacetic acid methyl ester | C3H6OS | 2 |
| 91 | 2,3-Butanediol | C4H10O2 | 5 |
| 93 | Toluene | C7H8 | 1, 7 |
| 95 | Dimethyl disulfide | C2H6S2 | 1, 2, 5 |
| 113 | 1-Octanol (fragment), octene | C8H16 | 2, 7 |
| 127 | 2,3-Dimethyl trisulfide | C2H6S3 | 2, 5, 7 |
| 127 | Nonene | C9H18 | 7 |
| 129 | 3-Ethyl-4-methyl hexane | C9H20 | 2 |
| 129 | Ethyl tiglate | C7H12O2 | 5 |
| 129 | Nonane | C9H20 | 7 |
| 129 | Octanone | C8H16O | 7 |
| 131 | Isoamyl acetate | C7H14O2 | 2, 7 |
| 131 | 1-Octanol | C8H18O | 5 |
| 143 | Dimethyl octane | C10H22 | 2 |
The VOCs were detected and tentatively identified by PTR-MS based on the protonated masses (m+1), fragmentation patterns, isotopic ratios, and published VOC compositions of spoiling meat.
Correlation between the VOC concentrations and the bacterial counts.
We calculated the Pearson product moment correlation coefficient rbm+1 for all measured VOC concentrations versus viable counts, Yb, for all five different groups of bacteria investigated (b = 1, total aerobic counts; b = 2, Pseudomonas spp.; b = 3, Enterobacteriaceae; b = 4, lactic acid bacteria; b = 5, Enterococcus spp.). We also calculated rbm+1 for the difference between the total viable counts and the viable counts for the other four groups of bacteria (b = 6; Y6 = Y1 − Y2 − Y3 − Y4 − Y5). Values for rbm+1 are shown in Table 3 for the masses listed in Table 2 with r > 0.7 for at least one bacterial group.
TABLE 3.
Calculated Pearson coefficients (rbm+1) for the correlation between the VOC concentrations detected on the basis of the protonated masses (m+1) and the numbers of the different groups of bacteria in air- and vacuum-packaged beef and pork stored over 11 days at 4°Ca
| m+1 (amu) | Meat | Packaging | Correlation coefficient between VOC concentrations and bacterial counts for:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Aerobic bacteria | Pseudomonas spp. | Enterobacteriaceae | Lactic acid bacteria | Enterococcus spp. | Other | |||
| 33 | Beef | Air | 0.89** | 0.81** | 0.82** | 0.47** | 0.60** | 0.02 |
| 49 | Beef | Air | 0.80** | 0.86** | 0.76** | 0.20 | 0.46** | −0.28 |
| 57 | Beef | Air | 0.69** | 0.73** | 0.66** | 0.58** | 0.57** | −0.22 |
| 63 | Beef | Air | 0.95** | 0.94** | 0.94** | 0.29 | 0.56** | −0.18 |
| 91 | Beef | Air | 0.85** | 0.92** | 0.93** | 0.21 | 0.56** | −0.34* |
| 93 | Beef | Air | 0.85** | 0.90** | 0.82** | 0.32 | 0.55** | −0.27 |
| 95 | Beef | Air | 0.76** | 0.84** | 0.77** | 0.19 | 0.37* | −0.32 |
| 113 | Beef | Air | 0.89** | 0.93** | 0.84** | 0.41* | 0.60** | −0.24 |
| 127 | Beef | Air | 0.82** | 0.91** | 0.80** | 0.24 | 0.48** | −0.34* |
| 129 | Beef | Air | 0.90** | 0.90** | 0.78** | 0.49** | 0.69** | −0.13 |
| 131 | Beef | Air | 0.88** | 0.86** | 0.74** | 0.40* | 0.66** | −0.09 |
| 143 | Beef | Air | 0.81** | 0.89** | 0.77** | 0.38* | 0.58** | −0.33 |
| 33 | Pork | Air | 0.88** | 0.84** | 0.87** | −0.12 | 0.71** | 0.57** |
| 63 | Pork | Air | 0.67** | 0.62** | 0.94** | −0.23 | 0.70** | 0.49** |
| 91 | Pork | Air | 0.61** | 0.57** | 0.88** | −0.20 | 0.59** | 0.44* |
| 95 | Pork | Air | 0.51** | 0.43** | 0.86** | −0.20 | 0.59** | 0.46** |
| 127 | Pork | Air | 0.79** | 0.76** | 0.86** | −0.18 | 0.72** | 0.52** |
| 47 | Beef | Vacuum | 0.60** | 0.54** | 0.31 | 0.72** | 0.69** | −0.21 |
| 49 | Beef | Vacuum | 0.69** | 0.66** | 0.43* | 0.73** | 0.70** | −0.18 |
| 91 | Beef | Vacuum | 0.83** | 0.64** | 0.54** | 0.66** | 0.66** | −0.04 |
∗, P < 0.05; ∗∗, P < 0.01 (n = 36).
(i) Comparison of air-packaged beef and pork.
For air-packaged beef we found the highest number of correlation coefficients (rbm+1 with values >0.7; Table 3). All rbm+1 values were below 0.7 for Enterococcus spp. and below 0.6 for lactic acid bacteria. That means that there were no VOCs in our detected spectra which had been produced by these two groups of bacteria. These two bacterial groups showed nearly no increase in their numbers over time (Fig. 1A). We also calculated the correlation between VOCs and the numbers of other bacteria (b = 6) to see whether the total number of viable bacteria contained species which were not investigated separately in this study but which produced some of the measured VOCs. We found all the correlation coefficients to be rather small (|r6m+1| < 0.4) and negative, i.e., only anticorrelations. That means that all the relevant bacterial groups contributing to our VOC spectra were determined in this study.
The rbm+1 values for air-packaged pork are listed in Table 3. There were only five VOCs for which rbm+1 was >0.7.
We got a smaller number of strong correlations (rbm+1 > 0.7) between VOCs and bacterial numbers for air-packaged pork than for air-packaged beef. The largest rbm+1 value (for both types of meat) was the one for the correlation for pork between the concentration of a VOC with an m+1 of 63 amu and the numbers of Pseudomonas spp. For beef, the strongest correlation for this VOC was with the total aerobic viable counts and this compound also showed a large rb63 for correlations with the numbers of Pseudomonas spp. and Enterobacteriaceae. Most of the strongest correlations between bacterial numbers and VOC concentrations for beef were with Pseudomonas spp., whereas the strongest ones for pork were with Enterobacteriaceae. We found the peak at an m+1 of 63 amu to be the most relevant peak for spoilage of air-packaged beef and pork in the present study.
(ii) Comparison of vacuum-packaged beef and pork.
For vacuum-packaged meat the number of correlations for which rbm+1 was >0.7 was small: three for beef (Table 3) and none for pork. As expected from a previous study (16) we obtained a strong correlation between the emission at an m+1 of 47 amu (assigned to ethanol) and the numbers of lactic acid bacteria. These bacteria are metabolically active under microaerophilic conditions and produce ethanol besides lactic acid. The number of bacteria at the end of the storage period (Fig. 1B and D) was too small to get a larger number of strong correlations. As we discuss below, reliable detection of smaller numbers of bacteria requires an increase of the measurement time of the PTR-MS system.
(iii) Calibration of the PTR-MS to determine bacterial contamination.
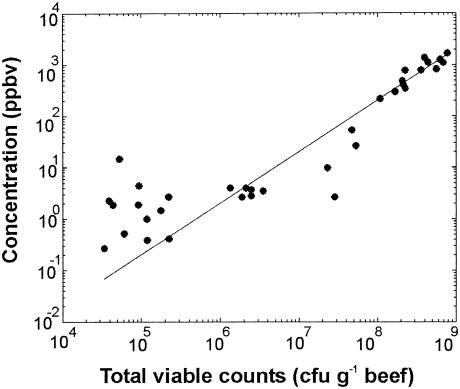
Our ultimate goal is to calculate the bacterial contamination by measuring the concentrations of few VOCs in the headspace air of a meat sample. To obtain the connection, we plotted the VOC concentration at a certain mass against the number of those groups of bacteria for which rbm+1 was >0.7 (Table 3). Then we calculated a linear fit to the data and determined its slope. An example for this procedure is shown in Fig. 5 where the concentration for an m+1 of 63 amu is plotted against the total counts for air-packaged beef. The solid line is a linear fit to the data.
FIG. 5.
Concentration of VOC for which m+1 was 63 amu versus the number of total aerobic viable bacteria in air-packaged beef. The solid line shows the linear fit to the measured data.
Monitoring the bacterial numbers.
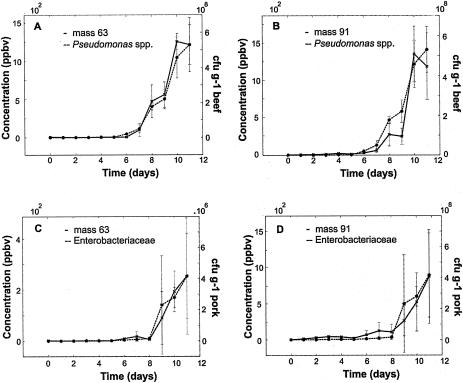
For monitoring bacterial numbers we first averaged over the three individually packaged meat samples taken on the same day to minimize random errors arising from various initial contaminations of different samples. Again, we compared the VOC concentrations and the bacterial counts by calculating the correlation coefficient rbm+1. Figure 6A shows the concentration for a VOC with an m+1 of 63 amu (typically emitted from spoiling air-packaged meat) and the total aerobic counts against time for air-packaged beef. The two curves have nearly the same shape. Averaging over the different replicates led to an increase in r163 from 0.95 (total counts) to 0.99 compared to the measurements described above. This indicates that we have no systematic errors. Making an on-line measurement of the VOC concentrations in the headspace air would allow bacterial growth in meat to be monitored. Some examples of the similar time developments of selected VOCs and bacterial counts are shown in Fig. 6B to D.
FIG. 6.
Mean concentrations of some VOCs (solid lines; see Table 2 for tentative identification) and mean bacterial numbers (dashed lines) in air-packaged beef (A and B) and pork (C and D). Each value is the mean for three individually packaged meat samples; the error bars indicate the standard deviations.
DISCUSSION
Meat tissue surfaces carry considerable bacterial loads. The initial meat contamination flora are very heterogeneous with respect to microbial numbers and composition (12, 24). The composition of the meat spoilage flora is greatly influenced by the storage conditions, such as temperature and type of packaging (18, 22). Packaging of chilled meat in gas-permeable materials (corresponding to air packaging in this study) does not alter the composition of the spoilage flora compared to that for unpackaged meat. The spoilage is dominated by gram-negative aerobic rod-shaped bacteria. Pseudomonas spp. are dominant (4, 6, 10). This was also shown in our study with air-packaged beef and pork. After 11 days of cold storage, the total aerobic population consisted almost entirely of pseudomonads; in addition we also observed a high concentration of Enterobacteriaceae.
The shelf life of meat is considerably increased by vacuum packaging instead of air packaging (14, 24). A large number of investigators have shown that, when O2-impermeable packaging is used, the growth of gram-positive bacteria, mostly lactic acid bacteria, is favored because of increased CO2 levels and a lowered oxidation-reduction potential (4, 10, 12, 18, 22, 23). These organisms typically cause a decrease in pH and create an unfavorable environment for most food-borne pathogens and gram-negative bacteria (12, 27). Under aerobic conditions, they cannot compete with gram-negative spoiling organisms due to much longer generation times (24). This was demonstrated in our study. Pseudomonads constituted only 9 to 18% of the total aerobic population after 11 days, compared to 83 to 100% in air-packaged meat. Gram-positive bacteria were almost negligible in air-packaged meat (≤0.02% of the total final population) but were present in considerable amounts in vacuum-packaged meat. Lactic acid bacteria and enterococci represented 51 and 43%, respectively, of the final population in beef, and 11 and 16%, respectively, of the microbial population in pork. Other authors have found comparable results when comparing bacterial numbers in air- and vacuum-packaged meat (3, 14, 17, 23, 25, 27)
As we already mentioned PTR-MS has some big advantages over the techniques usually used for gas analysis (e.g., gas chromatography). With PTR-MS no preparation of the samples, such as presampling, preconcentration, or sample dehydration, is necessary before the sample is admitted to the PTR-MS. Thus artifacts are avoided. Furthermore PTR-MS allows on-line measurements if necessary; however, for this study we did not need that capability since we were not interested in the time evolution of single meat samples. Another big advantage is that the absolute headspace concentrations can be calculated without calibration or use of standards. Since PTR-MS measurements are rapid, nondestructive, and quantitative, this system would be an ideal tool for fast bacterial analysis of meat.
To develop a fast method for the determination of the microbial spoilage status of meat, several steps are necessary. In the present study we proved and calculated the correlations between emitted VOCs and the microbial contamination by measuring them as a function of storage time simultaneously. We found very strong correlations, although we restricted ourselves to only a few compounds in this exploratory study. We plotted the VOC concentrations against the bacterial counts and calculated the slope of the linear fit through the data to get a conversion between these two parameters, as shown in Fig. 5. From the slope we can determine the numbers of total aerobic viable bacteria within minutes by measuring the concentration for a mass of 63 amu in the headspace air of a meat sample. In a corresponding bacteriological analysis it would take 48 h to obtain the results. Note that the linear fit shown in Fig. 5 is a very good approximation for high concentrations, whereas the data points with low concentrations have a large deviation. That is due to the absolute error in the PTR-MS measurements, which is described below. The ions reaching the detection system in the PTR-MS are described by a Poisson distribution. Therefore the relative error, e, of a PTR-MS measurement is equal to 1/c1/2 where c is the counts per counting time. c is proportional to the concentration of the VOCs times the primary ion density. There are two other sources of errors: dark counts and background concentrations. To get rid of these we subtracted the background concentration, which was measured before measuring the samples. This subtraction causes, however, additional errors for small concentrations due to the uncertainty in the number of the background ions. As an example, at a mass of 63 amu we had a background concentration of about 45 counts/s, corresponding to a concentration of 4 ppb by volume. As can be seen from Fig. 5 these background counts and the relative error in the remaining counts lead to a large total deviation for small numbers of bacteria. We could determine numbers of total aerobic viable bacteria exceeding 108 CFU g of meat−1 with an accuracy of about 99%. The numbers between 106 and108 CFU g−1 could be determined with an error of 30%, and below 106 CFU g−1 the error would be around 90%.
The relative error gets smaller with higher concentrations, longer counting times, or increasing numbers of primary ions. For instance, by increasing the counting time of our measurement from 0.2 to 5 s we can determine the total aerobic counts (Y1) >108 CFU g of meat−1 with an error of about 0.3%; in the range of 106 to 108 CFU g−1 the accuracy of 95%, and the error for counts between 104 and 106 CFU g−1 is approximately 20%.
In the long run, we anticipate that the presently explored method will enable the development of a fully automated quick method for monitoring the bacteriological contamination of meat based on the PTR-MS. Therefore a higher sensitivity in detecting low concentrations must be achieved to determine bacterial numbers lower than 106 CFU g−1 with sufficient accuracy (Fig. 5). The company Ionicon Analytik GmbH is currently developing PTR-MS instruments with a 5- to 10-times-higher primary ion signal, which enables the detection of lower concentrations. This method will have a wide range of applications: individual quality control in food markets, hygiene control in meat facilities (butchery), national quality control of meat distribution, gastronomy, and research. In trade it can be used for checking the quality of the open retailed meat in a supermarket by on-line measuring of the emissions in the storage area and for identifying contaminated meat pieces. There will be no need to destroy the meat cuts for the analysis, and one will get prompt information about the bacteriological contamination. Therefore one will be able to check a huge number of samples, and depending on the results meat samples will be sold or sorted out. For a meat-processing facility it is important to get meat of high quality with a low microbial contamination. Therefore one will be able to control the incoming meat with the PTR-MS method, which will help to decide if the meat should be bought and processed or not. Both applications will save a lot of money for the industry. The presently explored method enables meat controls in real time, which is required for national quality control that ensures the safety of food for the consumer. Another field of application is research: with PTR-MS one could observe the interaction of bacteria in vivo. The purchase costs are relatively high for a single supermarket or butchery but for big companies, national quality control institutions, universities, and research centers the PTR-MS technique could be very profitable.
Finally, we also want to mention that this method for measuring bacterial spoilage is not restricted to meat but can also be extended to food in general, for example, feed stuff, seafood, vegetables, and fruits.
Acknowledgments
We are grateful to Anton Mölk (head of the M-Preis, Innsbruck) for supporting this study by providing us with the meat samples.
This work was supported by the FWF and ÖNB, Vienna, Austria.
REFERENCES
- 1.Ahn, D. U., K. C. Nam, M. Du, and C. Jo. 2001. The effect of irradiation and packaging conditions on the formation of cholesterol and lipid oxidation products in meats during storage. Meat Sci. 57:419-426. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, D. U., C. Jo, and D. G. Olson. 2000. Analysis of volatile components and the sensory characteristics of irradiated raw pork. Meat Sci. 54:209-215. [DOI] [PubMed] [Google Scholar]
- 3.Blixt, Y., and E. Borch. 2002. Comparison of shelf-life of vacuum-packed pork and beef. Meat Sci. 60:371-378. [DOI] [PubMed] [Google Scholar]
- 4.Borch, E., M. L. Kant-Muermans, and Y. Blixt. 1996. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 33:103-120. [DOI] [PubMed] [Google Scholar]
- 5.Dainty, R. H., R. A. Edwards, C. M. Hibbard, and J. J. Marnewick. 1989. Volatile compounds associated with microbial growth on normal and high pH beef stored at chill temperatures. J. Appl. Bacteriol. 66:281-289. [DOI] [PubMed] [Google Scholar]
- 6.Dainty, R. H., and B. M. Mackey. 1992. The relationship between phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. Symp. Suppl. 73:103S-114S. [DOI] [PubMed]
- 7.Edwards, R. A., R. H. Dainty, and C. M. Hibbard. 1987. Volatile compounds produced by meat pseudomonads and related reference strains during growth on beef stored in air at chill temperatures. J. Appl. Bacteriol. 62:403-412. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, D. I., and R. Goodacre. 2001. Rapid and quantitative detection of the spoilage of muscle foods: current status and future trends. Trends Food Sci. Technol. 12:414-424. [Google Scholar]
- 9.Fox, S. 2001. WHO to convene on worldwide risk of BSE and vCJD. Infections in Medicine. 18:69. [Google Scholar]
- 10.Gill, C. O. 1986. The control of microbial spoilage in fresh meats, p. 49-88. In A. M. Pearson and T. R. Dutson (ed.), Advances in meat research: meat poultry microbiology. Macmillan, London, United Kingdom.
- 11.Hansel, A., A. Jordan, R. Holzinger, P. Prazeller, W. Vogel, and W. Lindinger. 1995. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Processes 149/150:609-619. [Google Scholar]
- 12.Jay, J. M. 1996. Modern food microbiology, 5th ed. Chapman & Hall, New York, N.Y.
- 13.King, M. F., B. L. Hamilton, M. A. Matthews, D. C. Rule, and R. A. Field. 1993. Isolation and identification of volatiles and condensable material in raw meat with supercritical carbon dioxide extraction. J. Agric. Food Chem. 41:1974-1981. [Google Scholar]
- 14.Lacroix, M., W. Smoragiewicz, M. Jobin, B. Latreille, and K. Krzystyniak. 2000. Protein quality and microbiological changes in aerobically- or vacuum-packaged, irradiated fresh pork loins. Meat Sci. 56:31-39. [DOI] [PubMed] [Google Scholar]
- 15.Lindinger, W., A. Hansel, and A. Jordan. 1998. On-line monitoring of volatile organic compounds at pptv level by means of proton-transfer-reaction mass spectrometry (PTR-MS). Int. J. Mass Spectrom. Ion Processes 173:191-241. [Google Scholar]
- 16.Mayr, D., R. Margesin, F. Schinner, and T. D. Märk. 2003. Detection of the spoiling of meat. Int. J. Mass Spectrom. Ion Processes 223/224:229-236. [Google Scholar]
- 17.Nissen, H., T. Maugesten, and P. Lea. 2001. Survival and growth of Escherichia coli O157:H7, Yersinia enterocolitica and Salmonella enteritidis on decontaminated and untreated meat. Meat Sci. 57:291-298. [DOI] [PubMed] [Google Scholar]
- 18.Nychas, G. J. E., E. Drosinos, and R. G. Board. 1998. Chemical changes in stored meat, p. 288-326. In R. G. Board and A. R. Davies (ed.), The microbiology of meat and poultry. Blackie, London, United Kingdom.
- 19.Pickrell, J., and M. Enserink. 2001. Foot-and-mouth disease—UK outbreak is latest in global epidemic. Science 291:1677. [DOI] [PubMed] [Google Scholar]
- 20.Stutz, H. K., G. J. Silverman, P. Angleini, and R. E. Levin. 1991. Bacteria and volatile compounds associated with ground beef spoilage. J. Food Sci. 56:1147-1153. [Google Scholar]
- 21.Tauxe, R. 2002. Emerging foodborne pathogens. Int. J. Food Microbiol. 78:31-41. [DOI] [PubMed] [Google Scholar]
- 22.Tsigarida, E., and G.-J. E. Nychas. 2001. Ecophysiological attributes of a Lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J. Appl. Microbiol. 90:696-705. [DOI] [PubMed] [Google Scholar]
- 23.Tsigarida, E., P. Skandamis, and G.-J. E. Nychas. 2000. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. J. Appl. Microbiol. 89:901-909. [DOI] [PubMed] [Google Scholar]
- 24.Upmann, M., P. Paulsen, S. James, and F. J. M. Smulders. 2000. The microbiology of refrigerated meat. Fleischwirtschaft 3:38-45. [Google Scholar]
- 25.Vold, L., A. Holck, Y. Waesteson, and H. Nissen. 2000. High levels of background flora inhibit growth of Escherichia coli O57:H7 in ground beef. Int. J. Food Microbiol. 56:219-225. [DOI] [PubMed] [Google Scholar]
- 26.Yeretzian, C., A. Jordan, R. Badoud, and W. Lindinger. 2002. From the green bean to the cup of coffee: investigating coffee roasting by on-line monitoring of volatiles. Eur. Food Res. Technol. 214:92-104. [Google Scholar]
- 27.Yost, C. K., and F. M. Nattress. 2002. Molecular typing techniques to characterize the development of a lactic acid bacteria community on vacuum-packaged beef. Int. J. Food Microbiol. 72:97-105. [DOI] [PubMed] [Google Scholar]