Figure 5.
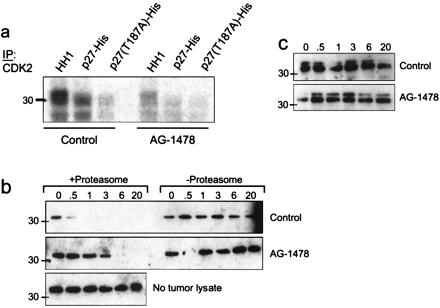
Phosphorylation/degradation of recombinant p27 by MMTV/Neu + TGF-α tumor lysates. (a) Cdk2 was precipitated from tumor lysates from mice that had been treated or not with AG-1478 and added to a kinase reaction containing either HH1, wild-type p27, or T187A p27 as substrates for [γ-32P]ATP incorporation. Phosphorylated products were resolved by SDS/PAGE and visualized by autoradiography. (b and c) Twenty micrograms of total protein from the tumor lysates was incubated with 250 ng of either wild-type p27 (b) or T187A p27 (c) at 30°C for the indicated times. Where indicated, tumor lysates were depleted of their proteasome complex by differential centrifugation before incubation with p27. To monitor for spontaneous degradation, wild-type p27-His was incubated under the same conditions but in the absence of a tumor lysate (b Bottom). Degradation was assessed by SDS/PAGE and p27 immunoblot as indicated in Materials and Methods. The endogenous p27 is undetectable by immunoblot analysis of this amount of total protein (20 μg) from the tumor lysates. Because of the histidine tag, both wild-type and mutant p27 molecules migrate as 33-kDa proteins.
