Figure 6.
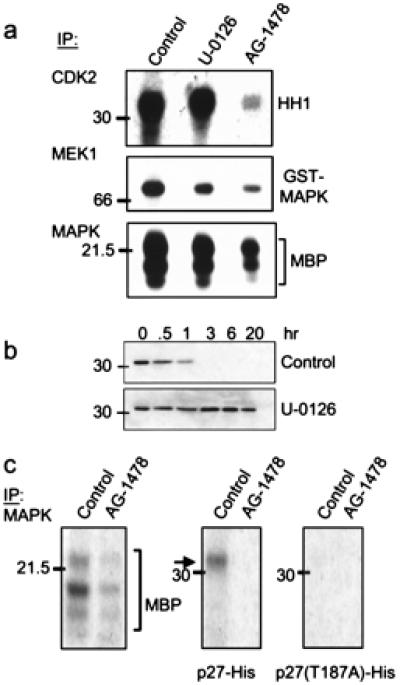
MAPK-mediated phosphorylation/degradation of p27. (a) Cdk2, MEK1, and MAPK were precipitated from a tumor lysate from a treatment-naïve mouse (control and U-0126 lanes) or from a mouse that had been treated with AG-1478 for 5 days. Immune complexes then were added to kinase reactions containing HH1, glutathione S-transferase-MAPK, or MBP, respectively. Where indicated, 10 μM U-0126 was added to the kinase reaction. Phosphorylated species were resolved by SDS/PAGE and visualized by autoradiography. (b) Twenty micrograms of total protein from a proteasome-containing tumor lysate was incubated with 250 ng of p27-His at 30°C for the indicated times in the presence of 10 μM U-0126 or DMSO (control). Degradation of p27 was monitored by immunoblot analysis as described in Fig. 5. (c) MAPK was precipitated from tumor lysates from mice that had been treated or not with AG-1478. Immune complexes then were tested for kinase activity against MBP, p27-His, or T187A p27 substrates. After SDS/PAGE, phosphorylated substrates were visualized by autoradiography.
