Figure 3.
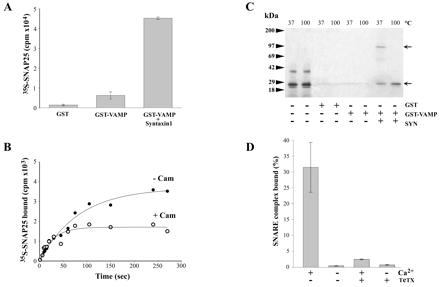
Inhibition of SNARE complex assembly by calcium/calmodulin. (A) In vitro translated 35S-SNAP-25 was incubated in the presence or absence of GST, GST-VAMP1–96, and untagged syntaxin 1A1–261 for 3 h at 4°C. Protein complexes were recovered on glutathione beads and, after washing, bound 35S-SNAP-25 was measured by β counting. Results are means ± SD, n = 3. (B) Samples prepared as in A were analyzed by SDS/PAGE and autoradiography after denaturation in SDS at 37°C or 100°C. Arrows indicate the migration of the trimeric core complex (upper arrow) and 35S-SNAP-25 (lower arrow). The radioactive band at about 40 kDa in the first two lanes is an unidentified translation product that did not interact with VAMP 2 or syntaxin 1A. (C) 35S-SNAP-25, GST-VAMP1–96, and syntaxin 1A1–261 were incubated at 4°C in a buffer containing 1 mM CaCl2, in the presence or absence of calmodulin (10 μM). At the indicated times, samples were removed, and bound 35S-SNAP-25 was evaluated by β counting. The curve is representative of three independent experiments. (D) SNARE assembly was performed as in A, except that complexes were recovered on calmodulin–agarose beads in the presence or absence of 1 mM CaCl2 and after pretreatment of GST-VAMP1–96 with TeTx light chain. 35S-SNAP-25 retained by calmodulin–agarose is shown as a percentage, taking the radioactivity recovered on glutathione beads as 100%, means + SD, n = 3.
