Figure 4.
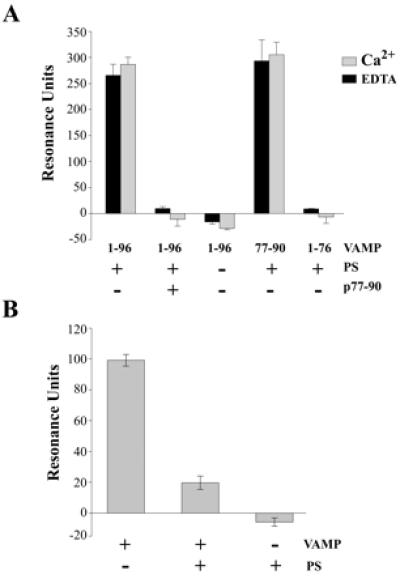
Interactions of phospholipids with the calmodulin-binding domain of VAMP 2. (A) GST-fusion proteins containing the indicated VAMP sequences were immobilized on an CM5 (Biacore) sensor chip coated with anti-GST antibodies. GST-VAMP1–76 was prepared from VAMP1–96 by TeTx cleavage. Liposomes containing 7.5 dipalmitoyl-PC: 2.5 dipalmitoyl-PS; weight: weight (PS+), or pure dipalmitoyl-PC (PS−), were diluted in running buffer and injected in the presence or absence of a synthetic peptide corresponding to VAMP 2 residues 77–94 (p77–94). Experiments were performed in the presence of 1 mM CaCl2 or 5 mM EDTA. Results are shown as means ± SD, n = 3. (B) Biotinylated calmodulin was immobilized on a streptavidin-coated sensorchip, and GST-VAMP1–96 was injected in the absence or presence of PC/PS liposomes. Control injections of liposomes were carried out in the absence of VAMP.
