Figure 5.
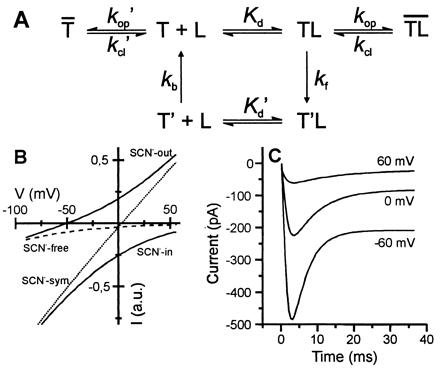
(A) Kinetic model for glutamate transport by EAAC1. Glutamate binding to the empty transporter, T, leads to the formation of the glutamate-bound state, TL. T′ and T′L are the respective states with the substrate binding site exposed to the cytoplasm. Charge translocation is assumed to be quasi-irreversible (zero-trans conditions). Na+ and K+ binding steps were assumed to be in rapid pre-equilibrium. (B) Simulation of steady-state anion and transport currents (Eqs. 3 and 4, Appendix). The following parameters were used: Kd = 50 μM, kf0 = 300 s−1, kb0 = 40 s−1, Φ1 = 20, Φ2 = 0.5, γ = 4 fS, z = −2, δ = 0.8. The conditions of the simulation were chosen similar to the experiments shown in Fig. 4 D and E. (C) Simulation of voltage-dependent currents as a function of time by numerical integration of the rate equations pertaining to the mechanism in A (conditions as in Fig. 4A). The rate constants for glutamate binding and dissociation were set to 2⋅107 M−1⋅s−1 and 1000 s−1, and kop and kcl were set to 1400 s−1 and 700 s−1, respectively. The other parameters were identical to those in B.
