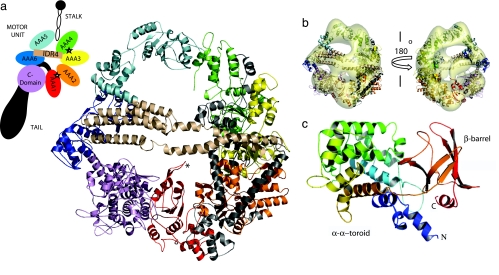
Fig. 1.
Model of the cytoplasmic dynein heavy chain. (a) Dynein consists of a motor unit (heavy chain) which generates force, a stalk that binds to microtubule, and a tail which oligomerizes with other dyneins. The atomic model of the motor unit contains six domains of the AAA enzyme family and a C domain. Domains are colored and identified according to schema. Active ATP-binding sites are indicated by stars. (b) Model structure from homology fitted to a 25-Å EM map of negatively stained cytoplasmic dynein (8) (EM map courtesy of M. Koonce). Motor unit consists of “smooth” (AAA1-AAA4) and “rough” (AAA5-C domain) sides. Also shown are the linkers between AAA1 and AAA2 (light gray), between AAA2 and AAA3 (black), between AAA3 and AAA4 (light gray), and between AAA5 and AAA6 (wheat). There are densities on the face of the ring. The long arching density putatively is formed by the coiled coil sequentially intermediate between AAA5 and AAA6, whereas the smaller density may be the remnant of the tail fragment. (c) Model of the C domain consists of an α–α toroid modeled from complement component C3d (PDB ID code 1GHQ) and a β-barrel modeled from leukemia-associated rhogef domain (PDB ID code 1TXD).