Abstract
Cystic tumors of the pancreas are less frequent than solid lesions and are often detected incidentally, as many of these lesions are small and asymptomatic. However, they may be associated with pancreatitis or have malignant potential. With advancements in diagnostic imaging, cystic lesions of the pancreas are being detected with increasing frequency. Many lesions can cause a pancreatic cyst, most being non-neoplastic while approximately 10% are cystic tumors, ranging from benign to highly malignant tumors. With increasing experience it is becoming clear that the prevalence of pseudocyst among cystic lesions of the pancreas is lower than usually presumed. A presumptive diagnosis of pseudocyst based on imaging appearance alone can cause a diagnostic error, and neoplastic cysts of the pancreas are particularly susceptible to this misdiagnosis, which can result in inappropriate treatment. Cystic tumors of the pancreas are formed by serous or mucinous structures showing all stages of cellular differentiation. According to the WHO classification, they can be subdivided on the basis of their histological type and biological behavior into benign tumors, borderline tumors, and malignant tumors. Cystic pancreatic tumors can be subdivided into peripheral (serous cystadenomas, mucinous cystic tumors, solid and papillary epithelial neoplasms, cystic islet cell tumors), which do not communicate with the main pancreatic duct, and ductal tumors (mucinous tumor), according to their site of origin. On the basis of imaging criteria alone, it can be very difficult to differentiate non-tumoral cystic lesions from neoplastic ones. The management of these patients is complex, and it is important to correlate imaging findings with knowledge of the patient’s symptoms and of the natural history and predictors of malignancy in pancreatic cysts.
Keywords: Pancreas; pancreatic neoplasms, diagnosis; cystadenoma, diagnosis; pancreas, pathology, radiography; neoplasms, cystic, mucinous; serous, diagnosis, pathology, radiography
Introduction
Cystic tumors of the pancreas occur with less frequency than solid lesions, and are often detected incidentally, as many of these lesions are small and asymptomatic. They may, however, be associated with pancreatitis or have malignant potential. With advancements in diagnostic imaging, cystic lesions of the pancreas are increasingly being detected; in some reference centers up to 30% of pancreatic resections are performed for cystic tumor . Accurate differentiation between cystic tumors is important because different treatments are required depending on the tumor’s histological type and grade. Due to frequent lack of specific clinical and laboratory signs and the overlap of imaging findings between different cystic tumors and between non-neoplastic and neoplastic cystic lesions of the pancreas , the management of these patients is complex. Knowledge of the patient’s symptoms and of the natural history and predictors of malignancy in pancreatic cysts is important.
When dealing with pancreatic cysts, the aim of imaging is to differentiate cystic tumors from tumor-like lesions and to characterize the cystic tumor, distinguishing benign tumor, which does not usually require surgical excision, from borderline or malignant tumor, which must be resected whenever possible. It can be very difficult to differentiate non-tumoral cystic lesions from neoplastic lesions on the basis of imaging criteria alone; in order to achieve a correct diagnosis it is important to correlate the imaging findings with the clinical history of the patient and the presence or absence of symptoms.
Many lesions can cause a pancreatic cyst . Most of these are non-neoplastic ; approximately 10% are cystic tumors, ranging from benign to highly malignant . Although all pancreatic tumors, including ductal adenocarcinoma, may appear as a cystic lesion as a result of degenerative changes , the classical cystic neoplasms of the pancreas are lined by different epithelium whose morphology constitutes the main diagnostic criterion and provides prognostic value, whereas their macroscopic appearance (shape, volume, etc.) has no importance .
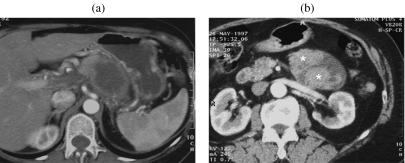
According to various authors, the majority (up to 90%) of cystic lesions of the pancreas seen in clinical practice are represented by post-inflammatory pseudocysts ; however, in a series of 212 consecutive patients with pancreatic cystic lesions observed in a reference center for pancreatic disease, pseudocysts represented only 13.6% of the lesions; referral bias cannot explain such a big difference . With increasing experience it is becoming clear that the prevalence of pseudocyst in cystic lesions of the pancreas is lower than previously thought . A presumptive diagnosis of pseudocyst based on imaging appearance alone can lead to a diagnostic error in up to 50% of cases , and neoplastic cysts of the pancreas are particularly susceptible to this misdiagnosis , resulting in inappropriate treatment (Fig. 1). In a comparison of incidental pancreatic cystic lesions , the incidence of pseudocyst was significantly higher in the group of symptomatic patients with a previous history of pancreatitis (26/29: 89.6%) than in the asymptomatic group (3/29: 10.4%). Thus, to make a confident diagnosis of pseudocyst it is necessary to correlate with the clinical history of the patient; previous chronic or severe acute pancreatitis is a useful predictor for the diagnosis of pseudocyst. However, in the same study, 25/42 (52%) of the patients with a history of pancreatitis had a cystic tumor of the pancreas ; thus previous pancreatitis cannot exclude the presence of a cystic tumor.
Figure 1.
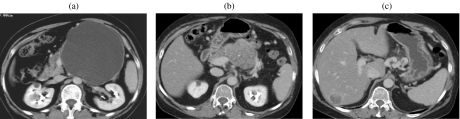
A 55-year-old woman with no history of pancreatitis. Mucinous cystic tumor. (a) At CT a diagnosis of ‘pseudocyst’ was made, and it was treated with cysto-entero anastomosis. Thirteen years later a solid mass (b) with a liver metastasis (c) can be appreciated.
From an imaging point of view, it is important to confirm or exclude a communication between the cystic lesion and the main pancreatic duct (m.p.d.). Cystic pancreatic tumors can be subdivided into peripheral (serous cystadenomas, mucinous cystic tumors, solid and papillary epithelial neoplasms, cystic islet cell tumors), which do not communicate with the m.p.d., and ductal tumors (mucinous tumor), according to their site of origin, although in a multi-institutional retrospective study a communication between the lesion and the pancreatic duct was discovered in 0.6% of those with serous cystadenoma, 6% of those with mucinous cystadenoma, and 10% of those with mucinous cystic adenocarcinoma .
Cystic tumors of the pancreas are formed by serous or mucinous structures showing all stages of cellular differentiation. According to the WHO classification , they can be subdivided on the basis of their histological type and biological behavior into benign tumors, borderline tumors, and malignant tumors .
Serous cystadenoma
Serous cystadenoma (SCA) occurs primarily in female patients in their fifties. It involves mainly the head of the pancreas, although it can be found in every part of the gland . Histologically, it is composed of multiple cysts formed by glycogen-rich, PAS-positive epithelial cells. It is a benign lesion, and no more than 10 cases of serous cystadenocarcinoma have been published .
It is important to obtain a certain diagnosis of SCA in a non-invasive way, as if the lesion is asymptomatic, it is possible to leave it unresected and to follow it up . A large lesion can be responsible for obstructive chronic pancreatitis, thus indicating a need for surgical resection (Fig. 2) .
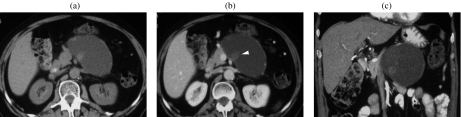
Figure 2.
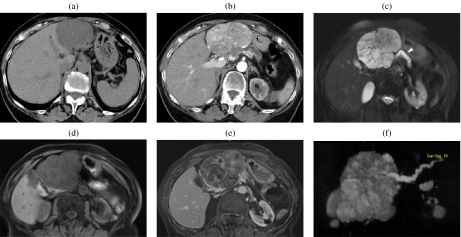
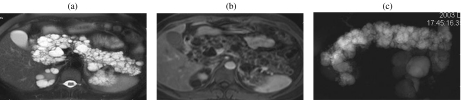
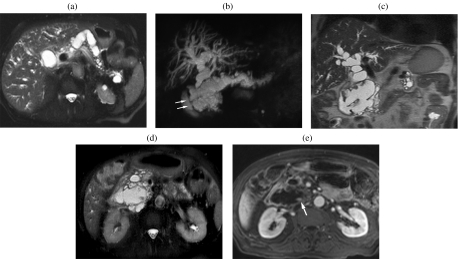
A 75-year-old woman with a serous cystic tumor. The patient complained of abdominal pain and a mass was palpable in the upper abdomen. At CT ((a), (b)) a large mass can be appreciated which shows a discrete enhancement after injection of CM (b). At MR ((c)–(f)) the lesion is hyperintense on T2w images (c), with microcystic appearance and significant dilation of the main pancreatic duct (arrowhead in (c)). With T1w images ((d), (e)) the lesion is hypointense with significant enhancement of the septa after CM injection (e). MRCP (f).
SCA is generally asymptomatic and is discovered incidentally during imaging studies performed for signs and symptoms not related to the pancreas. Symptomatic patients may complain of discomfort, while more serious symptoms due to larger lesions, such as jaundice, palpable mass and upper GI tract obstruction, are rare. In 15%–30% of cases, SCA is associated with von Hippel–Lindau (VHL) disease .
Imaging findings of SCAs are markedly dependent on their macroscopic appearance. Typically SCA has a microcystic appearance and presents as a well-circumscribed mass with multilobulate sharp margins and an internal sponge-like architecture due to the presence of innumerable microcysts (1–5 mm), seldom with rare larger cysts (>2 cm) peripherally located. A central stellate scar is typically visible, which can be calcified due to calcium deposits . These findings are recognized in about two-thirds of cases . Less frequently SCAs appear as a macrocystic or oligocystic mass, characterized by a small number of cysts > 2 cm which lack a central scar and are often indistinguishable from other cystic masses of the pancreas, especially mucinous cystic tumors . In microcystic form, at ultrasound SCAs appear as a mass with no posterior acoustic enhancement and a ‘honeycomb’ architecture (Fig. 3(a)). Due to the presence of thin-walled small cysts and a thick fibrous stroma, SCAs can be homogeneously hyperechoic at US, thus simulating a solid mass. At CT on unenhanced images SCA is hypodense (Figs. 2(a) and fig03(b)) or isodense to normal parenchyma, with a bulge in the contour of the gland; calcifications are well recognized, located in the center of the lesion. After contrast media administration, internal septa appear hyperdense due to contrast uptake, with the central fibrous scar visible (Figs. 2(b), (e) and fig03(c)–(e)). When SCA is small it can be difficult to characterize with CT, as it can appear as a solid mass (Fig. 3(b)–(d)); in this case, the great sensitivity of MR to static fluids with highly T2-weighted sequences permits demonstration of its fluid content (Fig. 3(e)), thus allowing accurate characterization. In fact, MR is assuming a preeminent role in the evaluation of cystic tumors of the pancreas, giving accurate information on the structure of the lesion (Figs. 2(c)–(e) and fig03(f)–(i)). Magnetic resonance cholangiopancreatography (MRCP) depicts the relationship of the lesion with the m.p.d. (Fig. 2(h)), thus permitting the differentiation between peripheral and intraductal cystic tumors. However, in some cases the close relationship between the cystic lesion and the m.p.d. does not exclude the presence of a communication (Figs. 3(i) and fig04); in these cases endoscopic retrograde cholangiopancreatography (ERCP) is still fundamental in the differential diagnosis (Fig. 4(b)). Contrast-enhanced MR gives the same information as CT, with clear demonstration of the enhancement of both the septa and the walls (Figs. 2(e) and fig03(h)). The main limitation of MR is its insensitivity to calcifications, which are better recognized with US or CT . When associated with VHL disease, cystic lesions are multifocal and can involve the pancreatic gland diffusely (Fig. 5).
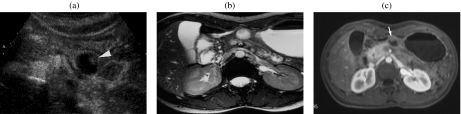
Figure 3.
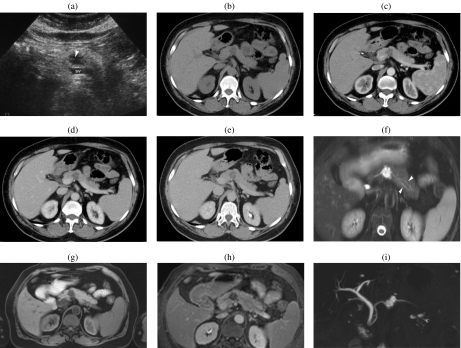
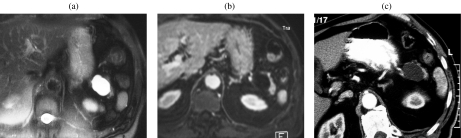
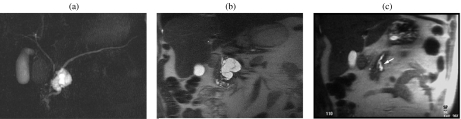
A 59-year-old woman with a serous cystic tumor. At US (a) a small hypoechoic lesion with no posterior acoustic enhancement is appreciable in the body of the pancreas. The lesion shows ‘honeycomb’ architecture. At CT in the unenhanced phase (b) the lesion appears hypodense with focal bulging of the pancreatic borders. After injection of iodinated contrast agent the lesion appears hypovascular in the arterial (c) and pancreatic (d) phase, showing enhancement of thin septa in the center of the lesion during the distribution phase (e). At MR the lesion is markedly hyperintense on T2 HASTE images, with microscystic appearance (f); in this sequence a branching of the main pancreatic duct in the tail can be observed (arrowheads). On T1w fat sat (g) the lesion appears hypointense with thin central septa which show enhancement after injection of paramagnetic contrast agent (h). MRCP (i) confirms the microcystic appearance; the close relationship between the cystic lesion and the m.p.d. does not allow exclusion of the presence of a communication. However, the microcystic appearance and the normal m.p.d. allow a correct diagnosis of serous cystic tumor.
Figure 4.
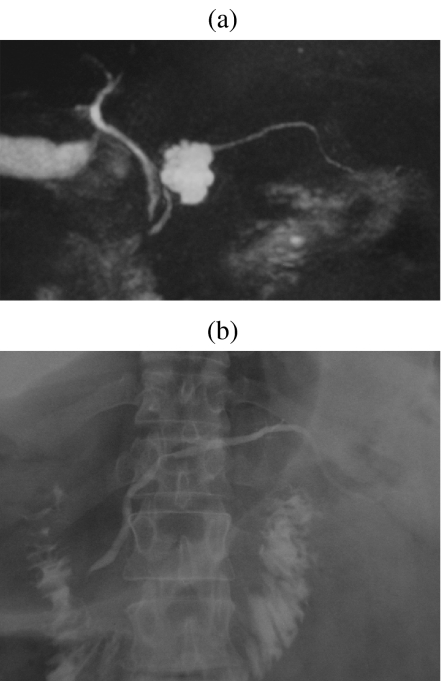
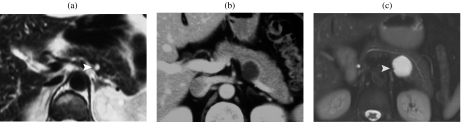
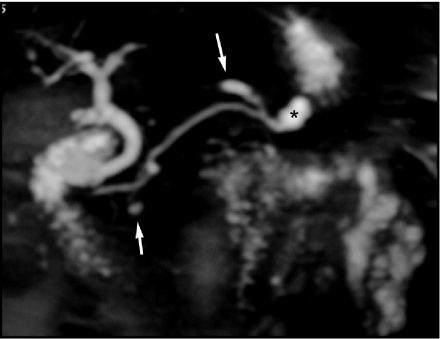
A 47-year-old man with a serous cystic tumor. At MRCP (a) the lesion shows microcystic appearance with unclear communication with the m.p.d. The ERCP (b) shows an impression in the m.p.d., but no communication, thus allowing a correct diagnosis of serous cystic tumor.
Figure 5.
A 25-year-old woman with von Hippel–Lindau disease. The pancreas appears completely substituted by multiple small cysts which appear hyperintense on T2w images (HASTE) (a). After injection of paramagnetic CM the thin septa among the cysts show a discrete enhancement (b). (c) MRCP.
Oligo- or macrocystic forms of SCA, on the other hand, have a nonspecific appearance which makes them indistinguishable from other cystic lesions of the pancreas in all imaging modalities (Figs 6 and 7).
Figure 6.
A 68-year-old man with a serous cystic tumor. At MR a macrocystic unilocular lesion is appreciable in the tail of the pancreas. The lesion is hyperintense on T2 HASTE (a), and does not show significant enhancement after injection of paramagnetic CM (b). (c) CT performed 5 years earlier: the lesion is unchanged.
Figure 7.
A 55-year-old woman with a mucinous cystic tumor. (a) At MR a small cyst in the body of the pancreas can be appreciated (arrowhead). (b) At CT performed 2 years later the cyst shows significant growth. (c) At MR 1 year later the lesion is further enlarged with macrocystic unilocular pattern and some small defects in the wall (arrowhead).
Mucinous cystic neoplasms
Mucinous cystic neoplasms (MCNs) are found almost exclusively in women. Histologically MCNs are formed by mucin-producing epithelial cells supported by an ovarian-type stroma . According to the grade of epithelial dysplasia, they can be classified into adenoma, border-line tumor or carcinoma, invasive or non-invasive . The peak age is the fifth decade, but a wide range of ages is affected. With advancing age the grade of the tumor worsens; this suggests an evolution from benign to malignant , as confirmed by the presence of the different degrees of differentiation, from benign to overtly malignant, in the same lesion .
MCN is located predominantly in the body-tail of the pancreas; when located in the head of the pancreas, mucinous cystadenocarcinoma is more prevalent . It is important to recognize this entity in an early phase of development, as prognosis of in situ carcinoma is much better than that of infiltrative carcinoma, which has a prognosis similar to that of ductal adenocarcinoma .
As for SCAs, symptoms for MCNs are nonspecific, with some degree of abdominal pain or discomfort, seldom referred to the pancreatic region. For advanced malignant lesions, clinical signs, such as dyspepsia, pain, weight loss and jaundice, may be more evident .
Imaging characteristics are again dependent on the macroscopic appearance. MCNs appear as a round mass with sharp margins with two main patterns, uni- or multilocular macrocysts . A macrocystic multilocular pattern is the most typical , while a unicystic appearance is similar to many other cystic lesions. In the absence of a history of pancreatitis, the correct diagnosis of a unicystic lesion with a thin wall is possible only after resection (Fig. 7) . At US multicystic MCN appears as a deeply hypoechoic mass with sharp margins; the wall has various thickness , with mural vegetation or parietal calcifications . Thick septa and/or papillary proliferations are suggestive of the malignant degeneration of MCN (Fig. 8(a)) . With contrast-enhanced US (CEUS), the wall and septa show variable degrees of enhancement (Fig. 8(b)). At unenhanced CT calcifications are clearly recognizable. The density of MCNs can vary from hypodense to hyperdense due to hemorrhage or mucin content . After CM injection either the wall (Fig. 9(a)) and the septa or solid component (Fig. 9(b)) show variable enhancement, although less than normal parenchyma . However, pseudocysts with necrotic debris, which can simulate parietal nodules, do not show significant enhancement after CM injection (Fig. 10(a)–(c)). The imaging triad of calcifications, thick walls, and mural vegetation is predictive of malignant degeneration in 95% of cases . MR with heavily T2-weighted sequences allows better identification of the thin septa than CT but has less sensitivity in identifying the calcifications. With MRCP the lack of communication between the MCN and the m.p.d. can be easily demonstrated, thus excluding an intraductal origin of neoplasia .
Figure 8.
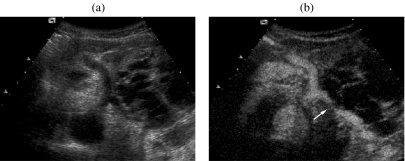
A 66-year-old woman with a mucinous cystic adenocarcinoma. At US (a) a large multilocular cyst with thick septa is appreciable in the body-tail of the pancreas. After injection of second-generation ultrasound contrast agent (Sonovue, Bracco, Italy) (b) the wall and septa show significant enhancement, with infiltration of the splenic artery (arrow). (Courtesy of Mirko D’Onofrio, MD.)
Figure 9.
Mucinous cystic adenocarcinoma, two different cases at CT. After injection of CM the thick wall (a) and the solid component (* in b) show significant enhancement.
Figure 10.
((a), (b)) Pseudocyst in a 70-year-old woman with a previous history of severe acute pancreatitis. A large unilocular cyst is appreciable in the tail of the pancreas with a solid component which does not show significant enhancement after injection of CM (arrowhead in (b)). (c) Coronal MPR.
As a differential diagnosis for the macrocystic pattern of SCA (Figs. 6 and 7), the cystic variant of solid pseudopapillary tumor (Fig. 11), the cystic endocrine tumor (Fig. 12) should be considered . Clinical history (MEN syndrome) or the evidence of hemorrhage inside the lesion (pseudopapillary tumor) can help in the diagnosis.
Figure 11.
A 28-year-old woman with a solid pseudopapillary tumor, cystic variant. At contrast-enhanced US (a) a small cystic lesion with sharp margins and an enhanced solid component (arrowhead) is appreciable in the body of the pancreas. With MR the lesion is hyperintense on T2w images (b) (True FISP), while the solid component shows enhancement after CM injection (arrow in (c)).
Figure 12.
A 47-year-old man with cystic endocrine tumor. At MR a large lesion with thick walls is appreciable in the head of the pancreas. The lesion is hyperintense on T2w (a) with significant dilation of the main pancreatic duct (arrow). After injection of paramagnetic CM a significant enhancement of the wall of the lesion can be appreciated (b).
Intraductal papillary mucinous tumor
Intraductal papillary mucinous tumor (IPMT) was first described in 1982 . In the last 10 years it has been increasingly described , thanks to the evolution of imaging modalities, especially MR, and to more widespread knowledge of this entity. In the past, many IPMTs were misdiagnosed as chronic pancreatitis. It has been classically described as a mucin-secreting tumor with dilation of the m.p.d. and a protruding papilla . IPMT can affect the m.p.d. and/or a branch-side duct. The tumor originates from the ductal epithelium and can evolve from slight dysplasia to infiltrating carcinoma over time with different aspects seen in the same lesion .
From the imaging point of view it is important not only to correctly differentiate IPMT from chronic pancreatitis, but also to suggest the histological grading of the tumor. In fact the only valid therapeutic option for IPMT is partial or total pancreatectomy, which in the case of a benign IPMT would amount to overtreatment, especially in branch-duct type . Thus, in order to achieve the correct management of these lesions, it is important to obtain all the clinical information and to correlate with imaging findings. IPMT affects patients in theirs 60s to 70s, with an equal distribution between men and women, while chronic pancreatitis is more frequent during the 40s . When located in the m.p.d. it is clinically characterized by recurrent abdominal pain simulating chronic pancreatitis , secondary to the obstruction of the pancreatic duct as a result of the hypersecretion of mucin . In a small percentage (<2%) of cases, IMPT can provoke an acute severe pancreatitis . In about 50% of cases patients complain of weight loss; in the early phase this is secondary to the pain evoked by food consumption while in the advanced phase it is secondary to the malignant degeneration of IPMT . About 10% of the patients suffer from diabetes, and the rapid onset of diabetes is highly suggestive of a malignant degeneration of IPMT . In a similar way, the onset of jaundice is suggestive of IPMT in an advanced stage . When IPMT is limited to side branch ducts it is almost always asymptomatic and becomes clinically evident when there is involvement of the m.p.d., with the same symptoms as an IPMT of the m.p.d.
There is common agreement that the only valid therapeutic option for IPMT of the m.p.d. as well as for side branch IPMT with involvement of the m.p.d., is surgical resection , as they are malignant at the time of the diagnosis in most of the cases. However, branch-duct type IPMT with a diameter < 3 cm, thin walls, and with no solid component or involvement of the m.p.d. can be followed-up , as most are asymptomatic, benign (hyperplasia-adenoma), and exhibit very slow growth .
IPMT of the m.p.d. can present as segmental or diffuse. When the entire duct is involved, this tumor can be confused with chronic pancreatitis . Segmental IPMT is very difficult to diagnose as it presents as a nonspecific segmental dilation of the m.p.d. without a clear cause of obstruction or a previous pancreatitis with post-inflammatory stenosis. The affected segment tends to enlarge with dilatation of the collateral ducts. When the lesion is localized in the head of the pancreas, there is a dilatation of the m.p.d. upstream, secondary to the obstruction of the outflow of pancreatic juice, thus simulating a diffuse IPMT . ERCP is able to characterize the lesion, with a clear demonstration of endoluminal defects and the possibility of aspirating the pancreatic juice in order to demonstrate the presence of mucin . Diffuse IPMT presents as a dilatation of the entire m.p.d. (Fig. 13(a), (b)) often indistinguishable from obstructive chronic pancreatitis . In diffuse IPMT there is frequent cystic dilation of branch ducts, especially in the uncinate process and in the tail of the pancreas ; moreover, in IPMT a bulging papilla into the duodenum can be observed, especially with CT or MR (Fig. 13(c)) after distension of the intestinal lumen with water or contrast agent . This latter observation is easily obtained with ERCP, which can also observe the mucin leaking into the papilla , making the diagnosis certain. However, in some cases ERCP is unable to opacify the m.p.d. owing to the reflux of contrast agent into the duodenum secondary to the patulous papilla or to the obstructive dense mucin, which can also hinder the opacification of branch ducts . MRCP can overcome the limitation of ERCP with a constant demonstration of branch ducts , thus giving an exact definition of the extent of IPMT. Both mucin and papillary growth appear hyperechoic at US; the solid component is better recognized at CT, especially after CM injection, or with MR, where it appears hypointense on T2-weighted MR sequences, showing enhancement after paramagnetic CM injection (Fig. 13(d), (e)) .
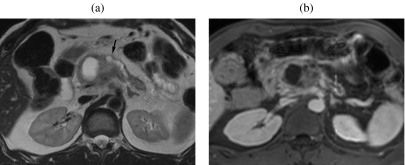
Figure 13.
An 83-year-old man with a history of abdominal pain and the onset of jaundice. Diffuse IPMT. On T2w images (a) a diffuse dilation of the main pancreatic duct is appreciable. With coronal MRCP (b) the dilation of the main pancreatic duct as well as of the biliary system can be depicted as a whole. A bulging of both major and minor papillae in the duodenal lumen can be appreciated (arrows), but is better depicted on coronal HASTE (c). At the uncinate process level a small defect in a dilated secondary duct can be seen (d), which shows significant enhancement after paramagnetic CM injection (arrow in (e)).
IPMT of the branch ducts is easily visualized as a cystic dilation of branch ducts (Fig. 14) and is increasingly diagnosed by chance during imaging studies performed for other reasons than the pancreas . They are more often located in the uncinate process, less frequently in the other portions of the pancreas, especially the tail . In 30% of cases IPMT is multifocal (Fig. 15). It appears as a round or oval lesion with multilobulated margins (Fig. 14). Cystic lesions converge in a ductal branch which communicates with the m.p.d.; this communication can be easily demonstrated with multislice CT by using thin collimation and is even better demonstrated with MRCP (Fig. 14(c)) . Sometimes the communication between the IPMT of the branch duct and the m.p.d. is not easily demonstrated; in such cases the administration of secretin with MRCP can facilitate the visualization of the communication, but sometimes even after secretin the communication cannot be demonstrated. In these cases ERCP can solve the diagnostic problem.
Figure 14.
A 75-year-old man with chance detection of pancreatic cyst at US performed as part of a routine check-up. Branch duct IPMT. With MRCP (a) a large plurilobular cyst in the head of the pancreas can be appreciated with normal m.p.d. ((b), (c)) Coronal HASTE: the lesion presents thin septa, no solid component, and a small communication with the m.p.d. can be appreciated (arrow in (c)).
Figure 15.
A 72-year-old man with recurrent episodes of acute pancreatitis. Segmental m.p.d and branch duct IPMT. MRCP. Branch duct dilations located at the head and tail of the pancreas (arrows). Mild segmental dilation of m.p.d. at the tail (asterisk).
Solid pseudopapillary tumor
Solid pseudopapillary tumor (SPT) is the least frequent cystic tumor of the pancreas whose origin is still uncertain. Since its first description in 1959, it has gone under different names and it was only in 1996 that the World Health Organization (WHO) renamed this tumor as SPT . It has a low malignant potential and a favorable prognosis. It may affect any age, but most often affects young women in their 30s . Clinically it is fairly nonspecific, with abdominal pain as the main symptom, sometimes together with a palpable mass. The low malignant potential of the lesion is demonstrated by its large size at the time of the diagnosis . The tumor appears as a round, well-encapsulated mass . The content of the lesion is solid with a variable amount of necrosis or hemorrhage, responsible for its frequent cystic appearance (Fig. 11). In a series of 56 patients with SPT, calcifications were found in 29% of the cases . After contrast agent administration the solid component of the tumor appears well vascularized with all imaging techniques (Fig. 11(a), (c)). When fresh hemorrhage is present, it is well recognized by MR, with high signal intensity on T1-weighted sequences and fluid–debris level . When large cystic changes are present, SPT appears as a macrocystic uni- or multilocular lesion, similarly to MCN; in this situation the young age of the patient helps in the differential diagnosis.
Conclusion
In conclusion, cystic tumors of the pancreas represent a diagnostic challenge for both radiologists and clinicians because of their overlapping clinical, radiological, and pathologic features. Although less prevalent than previously thought, pseudocysts are the most frequent cystic lesions of the pancreas, and a history of pancreatitis is an important factor in suggesting a diagnosis of pseudocyst, although it cannot exclude the coexistence of a pancreatic cystic tumor. The diagnosis of SCA can be considered certain when based on the finding of a microcystic or mixed (micro- and macrocystic) pattern; the diagnosis of either SPT (solid and fluid structure) or MCN (multilocular macrocystic pattern) is only presumptive, and all cystic tumors and tumor-like conditions may present a macrocystic uni- or multilocular pattern. Finally, even small cystic lesions of the pancreas appear to have the potential to progress to malignancy , and at least need follow-up with repeat imaging.
References
- 1.Salvia R, Festa L, Butturini G, Tonsi A, Sartori N, Biasutti C, et al. Pancreatic cystic tumors. Minerva Chir. 2004;59:185–207. [PubMed] [Google Scholar]
- 2.Compton CC. Histology of cystic tumors of the pancreas. Gastrointest Endosc Clin North Am. 2002;12:673–96. doi: 10.1016/s1052-5157(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Sarr MG. Cystic and pseudocystic diseases of the pancreas. Curr Probl Surg. 1994;31:165–243. doi: 10.1016/0011-3840(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 4.Kloppel G. Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 2000;17:7–15. [PubMed] [Google Scholar]
- 5.Hammel P, Bernades P. [Pancreatic cystic lesions of difficult diagnosis] Presse Med. 1996;25:1794–800. [PubMed] [Google Scholar]
- 6.Gasslander T, Arnelo U, Albiin N, Permert J. Cystic tumors of the pancreas. Dig Dis. 2001;19:57–62. doi: 10.1159/000050654. [DOI] [PubMed] [Google Scholar]
- 7.Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81–8. [PubMed] [Google Scholar]
- 8.Kloppel G, Longnecker D, Capella C, Sobin L. Berlin: Springer-Verlag; 1996. Histological Typing of Tumours of the Esocrine Pancreas. [Google Scholar]
- 9.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–33. doi: 10.1001/archsurg.138.4.427. discussion 433–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–53. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 11.Lumsden A, Bradley EL 3rd. Pseudocyst or cystic neoplasm? Differential diagnosis and initial management of cystic pancreatic lesions. Hepatogastroenterology. 1989;36:462–6. [PubMed] [Google Scholar]
- 12.Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas: new clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–43. doi: 10.1097/00000658-199010000-00006. discussion 444–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–61. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggi U, Di Candio G, Campatelli A, Pietrabissa A, Mosca F. Nonoperative management of pancreatic pseudocysts: problems in differential diagnosis. Int J Pancreatol. 1999;25:123–33. doi: 10.1385/ijgc:25:2:123. [DOI] [PubMed] [Google Scholar]
- 15.Civello IM, Frontera D, Viola G, Maria G, Crucitti F. Cystic neoplasm mistaken for pancreatic pseudocyst. Hepatogastroenterology. 1996;43:967–70. [PubMed] [Google Scholar]
- 16.Zamboni G, Capelli P, Pesci A, Brighenti A. Pathology of cystic tumor. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 9–30. [Google Scholar]
- 17.Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319–23. doi: 10.1007/s00268-002-6570-7. [DOI] [PubMed] [Google Scholar]
- 18.Widmaier U, Mattfeldt T, Siech M, Beger HG. Serous cystadenocarcinoma of the pancreas. Int J Pancreatol. 1996;20:135–9. doi: 10.1007/BF02825513. [DOI] [PubMed] [Google Scholar]
- 19.Eriguchi N, Aoyagi S, Nakayama T, et al. Serous cystadenocarcinoma of the pancreas with liver metastases. J Hepatobiliary Pancreat Surg. 1998;5:467–70. doi: 10.1007/s005340050075. [DOI] [PubMed] [Google Scholar]
- 20.Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–19. doi: 10.1097/01.sla.0000179651.21193.2c. discussion 419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann HP, Dinkel E, Brambs H, Wimmer B, Friedburg H, Volk B, et al. Pancreatic lesions in the von Hippel–Lindau syndrome. Gastroenterology. 1991;101:465–71. doi: 10.1016/0016-5085(91)90026-h. [DOI] [PubMed] [Google Scholar]
- 22.Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Guarise A, Valdo M, et al. Serous cystadenoma of the pancreas: report of 30 cases with emphasis on the imaging findings. J Comput Assist Tomogr. 1997;21:373–82. doi: 10.1097/00004728-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Carbognin G, Tapparelli M, Petrella A, Fuini A, Procacci C. Serous cystic tumor. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 31–55. [Google Scholar]
- 24.Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906–12. doi: 10.1097/00004728-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Nishihara K, Kawabata A, Ueno T, Miyahara M, Hamanaka Y, Suzuki T. The differential diagnosis of pancreatic cysts by MR imaging. Hepatogastroenterology. 1996;43:714–20. [PubMed] [Google Scholar]
- 26.Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–22. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumor of the pancreas: development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70:1505–13. doi: 10.1002/1097-0142(19920915)70:6<1505::aid-cncr2820700611>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Bassi C, Molinari E, Falconi M, Pederzoli P. Clinical manifestations and therapeutic management. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 3–8. [Google Scholar]
- 29.Sperti C, Cappellazzo F, Pasquali C, Militello C, Catalini S, Bonadimani B, et al. Cystic neoplasms of the pancreas: problems in differential diagnosis. Am Surg. 1993;59:740–5. [PubMed] [Google Scholar]
- 30.Biasiutti C, Fornasa F, Venturini S, Pagnotta N, Schenal G, Procacci C. Mucinous cystic tumors. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 57–74. [Google Scholar]
- 31.Fugazzola C, Procacci C, Bergamo Andreis IA, Iacono C, Portuese A, et al. Cystic tumors of the pancreas: evaluation by ultrasonography and computed tomography. Gastrointest Radiol. 1991;16:53–61. doi: 10.1007/BF01887305. [DOI] [PubMed] [Google Scholar]
- 32.Buetow PC, Rao P, Thompson LD. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic–pathologic correlation. Radiographics. 1998;18:433–49. doi: 10.1148/radiographics.18.2.9536488. [DOI] [PubMed] [Google Scholar]
- 33.Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 34.Procacci C, Carbognin G, Accordini S, Biasiutti C, Guarise A, Lombardo F, et al. CT features of malignant mucinous cystic tumors of the pancreas. Eur Radiol. 2001;11:1626–30. doi: 10.1007/s003300100855. [DOI] [PubMed] [Google Scholar]
- 35.Soyer P, Rabenandrasana A, Van Beers B, Barge J, Sibert A, Laissy JP, et al. Cystic tumors of the pancreas: dynamic CT studies. J Comput Assist Tomogr. 1994;18:420–6. doi: 10.1097/00004728-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Koito K, Namieno T, Ichimura T, Yama N, Hareyama M, Morita K, et al. Mucin-producing pancreatic tumors: comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology. 1998;208:231–7. doi: 10.1148/radiology.208.1.9646818. [DOI] [PubMed] [Google Scholar]
- 37.Mera K, Tajiri H, Muto M, Ohtsu A, Furuse J, Maru Y, et al. Clinical significance of magnetic resonance cholangiopancreatography for the diagnosis of cystic tumor of the pancreas compared with endoscopic retrograde cholangiopancreatography and computed tomography. Jpn J Clin Oncol. 1999;29:294–8. doi: 10.1093/jjco/29.6.294. [DOI] [PubMed] [Google Scholar]
- 38.Dupas B, Le Borgne J. [MRI-cholangiopancreatography characteristics of cystic pancreatic tumors] Ann Chir. 2000;125:571–7. doi: 10.1016/s0003-3944(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 39.Ohhashi K, Murakami Y, Takekoshi T, Ohta H, Ohhashi I. Four cases of mucus secreting pancreatic cancer. Prog Dig Endosc. 1982;20:348–51. [Google Scholar]
- 40.Falconi M, Salvia R, Bassi C, Zamboni G, Talamini G, Pederzoli P. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–81. doi: 10.1046/j.1365-2168.2001.01720.x. [DOI] [PubMed] [Google Scholar]
- 41.Azar C, Van de Stadt J, Rickaert F, Deviere M, Baize M, Kloppel G, et al. Intraductal papillary mucinous tumours of the pancreas: clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–64. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traverso LW, Peralta EA, Ryan JA Jr, Kozarek RA. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–32. doi: 10.1016/s0002-9610(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 43.Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–9. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Longnecker DS. Observations on the etiology and pathogenesis of intraductal papillary-mucinous neoplasms of the pancreas. Hepatogastroenterology. 1998;45:1973–80. [PubMed] [Google Scholar]
- 45.Rivera JA, Fernandez-del Castillo C, Pins M, Compton CC, Lewandrowski KB, Rattner DW, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms: a single malignant clinicopathologic entity. Ann Surg. 1997;225:637–44. doi: 10.1097/00000658-199706000-00001. discussion 644–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–6. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 47.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–7. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Cavallini G, Frulloni L, Pederzoli P, Talamini G, Bovo P, Bassi C, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scand J Gastroenterol. 1998;33:880–9. doi: 10.1080/00365529850171567. [DOI] [PubMed] [Google Scholar]
- 49.Kloppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology. 1998;45:1981–5. [PubMed] [Google Scholar]
- 50.Warshaw AL. Mucinous cystic tumors and mucinous ductal ectasia of the pancreas. Gastrointest Endosc. 1991;37:199–201. doi: 10.1016/s0016-5107(91)70688-3. [DOI] [PubMed] [Google Scholar]
- 51.Salvia R, Falconi M, Casetti L, Mantovani W, Frigerio I, Pederzoli P. Clinical manifestations and therapeutic management. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 77–84. [Google Scholar]
- 52.Wakabayashi T, Kawaura Y, Morimoto H, Watanabe K, Toya D, Asada Y, et al. Clinical management of intraductal papillary mucinous tumors of the pancreas based on imaging findings. Pancreas. 2001;22:370–7. doi: 10.1097/00006676-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Megibow AJ, Lombardo FP, Guarise A, Carbognin G, Scholes J, Rofsky NM, et al. Cystic pancreatic masses: cross-sectional imaging observations and serial follow-up. Abdom Imaging. 2001;26:640–7. doi: 10.1007/s00261-001-0024-9. [DOI] [PubMed] [Google Scholar]
- 54.Kimura W, Makuuchi M. Operative indications for cystic lesions of the pancreas with malignant potential—our experience. Hepatogastroenterology. 1999;46:483–91. [PubMed] [Google Scholar]
- 55.Kawarada Y, Yano T, Yamamoto T, Yokoi H, Imai T, Ogura Y, et al. Intraductal mucin-producing tumors of the pancreas. Am J Gastroenterol. 1992;87:634–8. [PubMed] [Google Scholar]
- 56.Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, et al. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19:1447–63. doi: 10.1148/radiographics.19.6.g99no011447. [DOI] [PubMed] [Google Scholar]
- 57.Tenner S, Carr-Locke DL, Banks PA, Brooks DC, Van Dam J, Farraye FA, et al. Intraductal mucin-hypersecreting neoplasm “mucinous ductal ectasia”: endoscopic recognition and management. Am J Gastroenterol. 1996;91:2548–54. [PubMed] [Google Scholar]
- 58.Procacci C, Carbognin G, Biasiutti C, Guarise A, Ghirardi C, Schenal G. Intraductal papillary mucinous tumors of the pancreas: spectrum of CT and MR findings with pathologic correlation. Eur Radiol. 2001;11:1939–51. doi: 10.1007/s003300100823. [DOI] [PubMed] [Google Scholar]
- 59.Dabezies MA, Campana T, Friedman AC. ERCP in the diagnosis of ductectatic mucinous cystadenocarcinoma of the pancreas. Gastrointest Endosc. 1990;36:410–11. doi: 10.1016/s0016-5107(90)71081-4. [DOI] [PubMed] [Google Scholar]
- 60.Kaneko T, Nakao A, Inoue S, Sugimoto H, Hatsuno T, Ito A, et al. Intraoperative ultrasonography by high-resolution annular array transducer for intraductal papillary mucinous tumors of the pancreas. Surgery. 2001;129:55–65. doi: 10.1067/msy.2001.109118. [DOI] [PubMed] [Google Scholar]
- 61.Fukukura Y, Fujiyoshi F, Sasaki M, Inoue H, Yonezawa S, Nakajo M. Intraductal papillary mucinous tumors of the pancreas: thin-section helical CT findings. AJR Am J Roentgenol. 2000;174:441–7. doi: 10.2214/ajr.174.2.1740441. [DOI] [PubMed] [Google Scholar]
- 62.Pavone E, Mehta SN, Hilzenrat N, Bret P, Lough J, Goresky CA, et al. Role of ERCP in the diagnosis of intraductal papillary mucinous neoplasms. Am J Gastroenterol. 1997;92:887–90. [PubMed] [Google Scholar]
- 63.Itai Y, Kokubo T, Atomi Y, Kuroda A, Haraguchi Y, Terano A. Mucin-hypersecreting carcinoma of the pancreas. Radiology. 1987;165:51–5. doi: 10.1148/radiology.165.1.3306789. [DOI] [PubMed] [Google Scholar]
- 64.Arakawa A, Yamashita Y, Namimoto T, Tang Y, Tsuruta J, Kanemitsu K, et al. Intraductal papillary tumors of the pancreas: histopathologic correlation of MR cholangiopancreatography findings. Acta Radiol. 2000;41:343–7. doi: 10.1080/028418500127345622. [DOI] [PubMed] [Google Scholar]
- 65.Irie H, Honda H, Aibe H, Kuroiwa T, Yoshimitsu K, Shinozaki K, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000;174:1403–8. doi: 10.2214/ajr.174.5.1741403. [DOI] [PubMed] [Google Scholar]
- 66.Cellier C, Cuillerier E, Palazzo L, Rickaert F, Flejou JF, Napoleon B, et al. Intraductal papillary and mucinous tumors of the pancreas: accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998;47:42–9. doi: 10.1016/s0016-5107(98)70297-4. [DOI] [PubMed] [Google Scholar]
- 67.Lim JH, Lee G, Oh YL. Radiologic spectrum of intraductal papillary mucinous tumor of the pancreas. Radiographics. 2001;21:323–37. doi: 10.1148/radiographics.21.2.g01mr01323. discussion 337–40. [DOI] [PubMed] [Google Scholar]
- 68.Procacci C, Schenal G, Dalla Chiara E, Fuini A, Guarise A. Intraductal papillary mucinous tumor: imaging. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 97–137. [Google Scholar]
- 69.Kloppel G, Morohoshi T, John HD, Oehmichen W, Opitz K, Angelkort A, et al. Solid and cystic acinar cell tumour of the pancreas: a tumour in young women with favourable prognosis. Virchows Arch A Pathol Anat Histol. 1981;392:171–83. doi: 10.1007/BF00430819. [DOI] [PubMed] [Google Scholar]
- 70.Megibow A, Francis IR. Unusual pancreatic neoplasms: Imaging. In: Procacci C, Megibow A, editors. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer; 2003. pp. 249–65. [Google Scholar]
- 71.Ng KH, Tan PH, Thng CH, Ooi LL. Solid pseudopapillary tumour of the pancreas. ANZ J Surg. 2003;73:410–15. doi: 10.1046/j.1445-2197.2003.t01-1-02634.x. [DOI] [PubMed] [Google Scholar]
- 72.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging–pathologic correlation on 56 cases. Radiology. 1996;199:707–11. doi: 10.1148/radiology.199.3.8637992. [DOI] [PubMed] [Google Scholar]
- 73.Procacci C, Graziani R, Bicego E, Zicari M, Bergamo Andreis IA, Zamboni G, et al. Papillary cystic neoplasm of the pancreas: radiological findings. Abdom Imaging. 1996;21:554–8. [PubMed] [Google Scholar]
- 74.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]