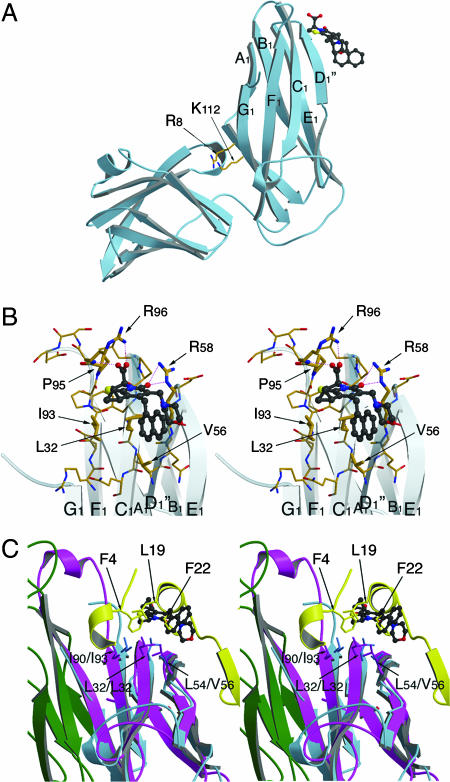
Fig. 3.
Structure of the PapD–2c complex. (A) Side view from the PapD chaperone showing the pilicide-binding side at the N-terminal domain, near the F1–G1 loop. The conserved cleft residues R8 and K112 are indicated as a point of reference. In the PapD–2c crystals, no pilicide is seen binding in the cleft; rather, the R8/K112 pair is involved in crystal packing (data not shown). (B) Stereoimage of the pilicide-binding site near the F1–G1 loop. The 2c compound, shown in ball-and-stick representation, forms hydrogen bond and electrostatic interactions with the R96, the P95-R96 backbone amide and R58. Further interactions are formed with the hydrophobic patch made up of the residues I93, L32, and V56. (C) Stereoimage of the PapD–2c complex in overlay with the FimD1–125 N-terminal usher domain in complex with the FimC-FimH158–279 chaperone–adhesin complex. PapD and 2c are shown in light blue ribbon and ball-and-stick representation, respectively. FimC and FimH158–279 are shown in magenta and green, with the FimD1–125 N-terminal domain shown in yellow. The conserved hydrophobic patch across the back of the F1-C1-D1 β-sheet formed by residues I90/I93, L32/L32, and L54/V56 (FimC/PapD) forms part of both the usher-interaction site and the pilicide-binding site. The overlay shows how in the chaperone–pilicide interaction, the plane of the 2-pyridone system and the R1-cyclopropyl and R2-CH2-naphthyl substituents coincide with and mimic the interactions made by the F4, L19, and F22 side chains from the usher N-terminal domain. Finally, the overlay clearly demonstrates the steric clash between the pilicide and the usher N-terminal domain. Figures were generated with Molscript and Raster3D (31, 32).