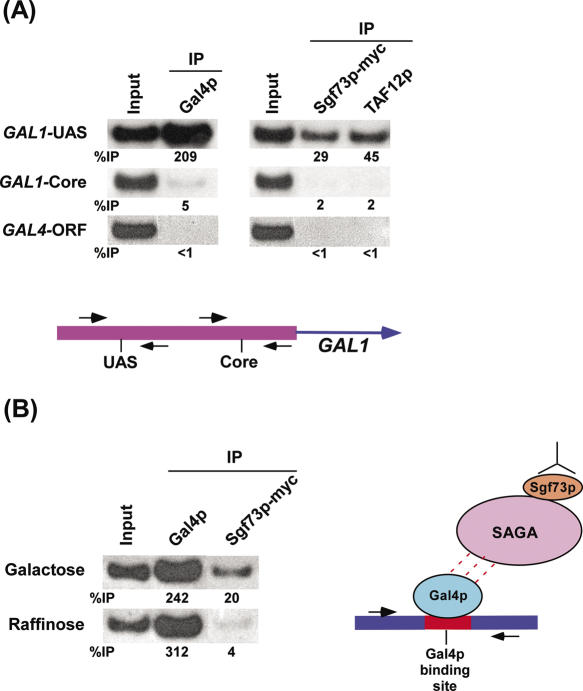
Figure 1.
Recruitment of Sgf73p to the GAL1 UAS or minimal Gal4p binding site. (A) Recruitment of Sgf73p to the UAS, but not core promoter, of the GAL1 gene. Yeast strain expressing myc-epitope tagged Sgf73p was grown at 30°C in 1% yeast extract containing 2% peptone plus 2% galactose (YPG) up to an OD600 of 1.0 prior to formaldehyde-based in vivo crosslinking. The chromatin (IP) was performed as previously described (23,24). Primer-pair located at the UAS or core promoter of the GAL1 gene (see Materials and Methods section) was used for PCR analysis of the (IP) DNA samples. A PCR fragment corresponding to the GAL4 ORF was used as a control for background binding. (IP) was performed using a mouse monoclonal antibody against the c-myc epitope-tag (9E10; Santa Cruz Biotechnology, Inc.) or a polyclonal antibody against TAF12p (from Michael R. Green, University of Massachusetts Medical School). A mouse monoclonal antibody against the DNA binding domain of Gal4p (RK5C1; Santa Cruz Biotechnology, Inc.) was used. The primer-pairs are indicated on the left. The ratio of immunoprecipitate over the input is indicated below each band as %IP. (B) Recruitment of Sgf73p to the minimal Gal4p-binding sites. The plasmid containing minimal Gal4p-binding sites was transformed into the yeast strain expressing myc-epitope tagged Sgf73p. Yeast strain thus generated, was grown at 30°C in 1% yeast extract containing 2% peptone plus 2% galactose or raffinose. The ChIP analysis was performed as described in (A). The primers used for the PCR analysis are adjacent to the Gal4p-binding sites in the plasmid (see Materials and Methods section). The growth media are indicated on the left.